Page 1 :
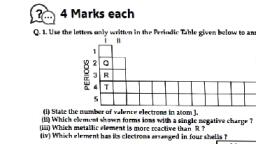
ASSIGNMENT, Total Marks : 20, I. Multiple Choice Questions, (1 Mark), Choose the correct answer from the given options., 1. An element X' with atomic number 12 forms a compound with element 'Y with atomic number 17. The, formula of the compound is, (b) XY,, (d) X,Y,, (a) XY, [CBSE 2020], (c) X,Y, 2. An element X is forming acidic oxide. Its most probable position in the modern periodic table is, (a) Group 1 and Period 3, (e) Group 17 and Period 3, II. Assertion-Reason Type Questions, Note: Use instructions as given in topical exercises of the chapter., 1. Assertion: A. X' has mass no. 35, number of neutrons are 18., Reason: X belongs to Group 17 and 3rd period, it is non-metal, 2. Assertion: Out of A(4), B(9), C(14), D(19), E(20), D(19) has one valence electron., Reason: Valency of A(4) and E(20) are equal to 2., III. Very Short Answer Type Questions, (b) Group 16 and Period 3, (d) Group 2 and Period 3, [CBSE 2020], (1 Mark), (1 Mark), 1. If the atomic mass of 'Na' is 23 and K' is 39, calculate atomic mass of Li, if these elements form, Dobereiners' triads., 2. An element belongs to Group 15, 2nd period. Write down the formula of its oxide and hydride., IV. Short Answer Type Questions-l, (2 Marks), 1. How does tendency to lose electrons change in a period and why?, 2. How does ionic size vary down the group and along a period from left to right? Why?, 3. What is a metalloid and where are they positioned in the periodic table?, V. Short Answer Type Question-I|, 1. From the elements Li, K, Mg, C, Al and S identity, (a) the case elements belonging to the same group., (b) element which has the tendency to lose two electrons, (c) element which prefers sharing of electrons to complete its octet., (3 Marks), (d) most metallic element, (e) element that forms acidic oxide, ) element that belongs to group 13, [CBSE 2020], VI. Long Answer Type Question, 1. Answer the following questions based on elements with atomic number 3 to 9., (5 Marks), (a) Name the element which has smallest atomic radius., (b) Name the element which shows maximum valency., (c) Name the element which is metalloid., (d) Name the element which is most electropositive., (e) Write the chemical formula of a compound formed when the element with atomic number 6 and 8, react together.