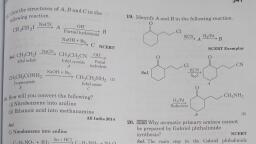
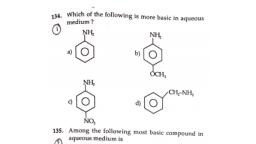
Page 1 :
Exercises, , 13.1 Write IUPAC names of the following compounds and classify them into primary,, secondary and tertiary amines., , () (CH,),CHNH, (ii) CH,(CH,),NH, (iii), CH,NHCH(CH,),, (iv) (CH,),CNH, (v) C,H,NHCH, (vi) (CH,CH,),NCH,, (vii) m-BrC,H,NH,, 13.2 Give one chemical test to distinguish between the following pairs of compounds., (i) Methylamine and dimethylamine (ii) Secondary and tertiary amines, (iii) Ethylamine and aniline (iv) Aniline and benzylamine, (v) Aniline and N-methylaniline., 13.3 Account for the following:, (i) pK, of aniline is more than that of methylamine., (ii) Ethylamine is soluble in water whereas aniline is not., , (iii) Methylamine in water reacts with ferric chloride to precipitate hydrated, ferric oxide., , (iv) Although amino group is o- and p- directing in aromatic electrophilic, substitution reactions, aniline on nitration gives a substantial amount of, m-nitroaniline., , (v) Aniline does not undergo Friedel-Crafts reaction., , (vi) Diazonium salts of aromatic amines are more stable than those of aliphatic, amines., , (vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines., , 13.4 Arrange the following:, , (i) In decreasing order of the pK, values:, , C,H,NH,, C,H,NHCH,, (C,H,),NH and C,H,NH,, , (ii) In increasing order of basic strength:, , CH NH,, CsH,N(CH,),, (C,H,),.NH and CH,NH,, , (iii) In increasing order of basic strength:, , (a) Aniline, p-nitroaniline and p-toluidine, , , , hemistry (408,, , (iv), , (v), , (vi), , 2021-22, , (b) CsH3NH,, C,H;NHCH,, C,H,CH,NH,., , In decreasing order of basic strength in gas phase:, C,H,NH,, (C,H,),NH. (C,H,);N and NH,, , In increasing order of boiling point:, , C,H,OH, (CH,),NH, C,H,NH,, , In increasing order of solubility in water:, , C.H;NH,, (C,H,),NH, C,H,NH,., , 3.5 How will you convert:, , @i, (iil, (ii), (iv), v), (vi), (vii), (viii, , Ethanoic acid into methanamine, Hexanenitrile into 1-aminopentane, Methanol to ethanoic acid, Ethanamine into methanamine, Ethanoic acid into propanoic acid, Methanamine into ethanamine, Nitromethane into dimethylamine, Propanoic acid into ethanoic acid?, , 3.6 Describe a method for the identification of primary, secondary and tertiary amines., Also write chemical equations of the reactions involved., , 3.7 Write short notes on the following:, , @i, (iii), , (v, (vii, , Carbylamine reaction (ii) Diazotisation, Hofmann’s bromamide reaction (iv) Coupling reaction, Ammonolysis (vi) Acetylation, , Gabriel phthalimide synthesis., , .3.8 Accomplish the following conversions:, , , , fi, , , , Nitrobenzene to benzoic acid