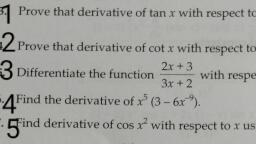Question 1 :
If the atomic number of an element is {tex} 33 , {/tex} it will be placed in the periodic table in the
Question 2 :
Which of the following sequence correctly represents the decreasing acidic nature of oxides?
Question 4 :
Which one of the following ionic species has the greatest proton affinity to form stable compound?
Question 6 :
Aqueous solutions of two compounds {tex} \mathrm { M } _ { 1 } - \mathrm { O } - \mathrm { H } {/tex} and {tex} \mathrm { M } _ { 2 } - \mathrm { O } - \mathrm { H } {/tex} are prepared in two different beakers. If, the electronegativity of {tex} \mathrm { M } _ { 1 } = 3.4 {/tex} {tex} \mathrm { M } _ { 2 } = 1.2 , \mathrm { O } = 3.5 {/tex} and {tex} \mathrm { H } = 2.1 {/tex} then the nature of two solutions will be respectively:
Question 7 :
Sodium sulphate is soluble in water whereas barium sulphate is sparingly soluble because :
Question 8 :
Which property decreases from left to right across the periodic table and increases from top to bottom?<br>(i) Atomic radius {tex} \quad\quad\quad\quad\quad {/tex}(ii) Electronegativity <br> (iii) Ionisation energy{tex} \quad\quad\quad {/tex} (iv) Metallic character<br>
Question 10 :
The electron affinity of chlorine is {tex} 3.7 \mathrm { eV } \ .1 \mathrm { g } {/tex} of chlorine is completely converted to {tex} \mathrm { Cl } ^ { - } {/tex} ion in a gaseous state. {tex} \left( 1 \mathrm { eV } = 23.06 \ \mathrm { kcal } \ \mathrm { mol } ^ { - 1 } \right) . {/tex} Energy released in the process is
Question 11 :
For alkali metals, which one of the following trends is incorrect?
Question 12 :
{tex} \mathrm { Na } ^ { + } , \mathrm { Mg } ^ { 2 + } , \mathrm { Al } ^ { 3 + } {/tex} and {tex} \mathrm { Si } ^ { 4 + } {/tex} are isoelectronic. the order of their ionic size is
Question 13 :
Similarity in chemical properties of the atoms of elements in a group of the Periodic table is most closely related to:
Question 16 :
The first ionization potentials {tex} ( \mathrm { eV } ) {/tex} of {tex} \mathrm { Be } {/tex} and {tex} \mathrm { B } {/tex} respectively are
Question 18 :
Generally, the first ionisation energy increases along a period. But there are some exceptions. One which is not an exception is
Question 19 :
Which is the correct order of second ionization potential of {tex} \mathrm { C } , \mathrm { N } , \mathrm { O } {/tex} and {tex} \mathrm { F } {/tex} in the following?
Question 20 :
Ionization energies of atoms A and B are {tex} 400{/tex} and {tex}300\mathrm { \ k \ cal \ mol}^{-1} {/tex}respectively. The electron affinities of these atoms are {tex} 80.0{/tex} and {tex}85.0\mathrm { \ k \ cal \ mol}^{-1} {/tex} respectively. Then which is the correct statement regarding electronegativity {tex}\chi{/tex}?