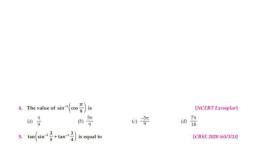Question 1 :
The radius of a sphere is measured to be $5.3 \pm 0.1 cm$. Calculate the percentage error in the measurement of its volume.
Question 2 :
A public park, in the form of a square, has an area of $(100 \pm 0.2 )m^2 $ .The side of park is :
Question 3 :
A scientist proposes a new temperature scale in which the ice point is $25 X$ ($X$ is the new unit of temperature) and the steam point is $305 X$. The specific heat capacity of water in this new scale is (in $J{ kg }^{ -1 }{ X }^{ -1 }$):
Question 4 :
Two equal masses each $'m'$ are hung from a balance whose scale pans differe in vertical height by $'h'$. the error in weighing is
Question 5 :
Match the following physical quantities with their respective dimensional formula:<br/><table class="wysiwyg-table"><tbody><tr><td>(a) Angular Momentum</td><td>(e) $\displaystyle \left[ { ML }^{ 2 }{ T }^{ -3 } \right] $</td></tr><tr><td>(b) Impulse</td><td>(f) $\displaystyle \left[ { ML }^{ 2 }{ T }^{ -1 } \right] $</td></tr><tr><td>(c) Pressure</td><td>(g) $\displaystyle \left[ ML{ T }^{ -1 } \right] $</td></tr><tr><td>(d) Power</td><td>(h) $\displaystyle \left[ { ML }^{ -1 }{ T }^{ -2 } \right] $</td></tr></tbody></table>
Question 6 :
The Van der Waal's equation of $'n'$ moles of a real gas is<br/>$\displaystyle \left( P+\frac { a }{ { V }^{ 2 } }  \right) \left( V-b \right) =nRT$<br/>Where $P$ is pressure, $V$ is volume, $T$ is absolute temperature, $R$ is molar gas constant and $a, b, c$ are Van der Waal constants. The dimensional formula for $ab$ is:
Question 7 :
The period of oscillation of a simple pendulum is $\displaystyle T = 2 \pi \sqrt {\dfrac {L} {g}}$. Measured of $L$ is $20.0\ cm$ known to $1\ mm$ accuracy and time for $100$ oscillations of the pendulum is found to be $90\ s$ using a wrist watch of $1\ s$ resolution. The accuracy in the determination of $g$?
Question 8 :
The mass of a box measured by a grocer's balance is $2.3\ kg$. two gold pieces of masses $20.15\ g$ and $20.17\ g$ are added to the box. The total mass of the box is:
Question 9 :
The speed of light ($c$), gravitational constant ($G$) and Planck's constant ($h$) are taken as fundamental units in a system. The dimensions of time in this new system should be
Question 10 :
The numbers 3.845 and 3.835 on rounding off to 3 significant figures will give then<br/>
Question 11 :
The pressure of the gas was found to decrease from $720$ to $480\ mm$. When $5\ g$ of the sample of activated charcoal was kept in a flask of one litre capacity maintained at $27^{\circ}C$. If the density of charcoal at $1.25\ gm/ mL$. The volume of gas adsorbed per gm of charcoal at $480\ mm$ of $Hg$ is:
Question 12 :
The radius of a ball is $(5.4\pm 0.2)cm$. The percentage error in the volume of the ball is
Question 13 :
The viscosity of a gas depends on mass, the effective diameter and the mean speed of the molecules. At room temperature, for He, $\eta_{He}=2\times 10^{-5}$ $kg{m}^{-1}{s}^{-1}$ and for $CH_{4}$, $\eta _{CH_{4}}=1.1\times 10^{-5}kgm^{-1}s^{-1}$. The diameter of the He atom is $2.1\times 10^{-10}m$ . lf the diameter of  $CH_{4}$ is $n\times 10^{-10}m$, find $'n'$.<br/>Given mean speed of the molecules of the gas $v\propto \sqrt{\frac{K_B T}{m}}$ , where $K_B$ is the boltzmann's constant , $ T$ is temperature and m is the mass of the gas. 
Question 14 :
In an experiment the following observations were recorded <br/>$L=2.890$ metre<br/>$\mathrm{M}=3.0$ kg<br/>$\ell=0.87$ cm<br/>$\mathrm{D}=0.041$ cm<br/>$\mathrm{g}=981\mathrm{c}\mathrm{m}/\sec^{2}$<br/>and the formula used for calculatlon of Young's modulus ($\mathrm{Y}$) is $\displaystyle \mathrm{Y}=\frac{\mathrm{M}\mathrm{g}\mathrm{L}}{\pi \mathrm{r}^{2}l}$<br/>What is the maximum possible error expressed in percentage?<br/>
Question 15 :
<table class="wysiwyg-table"> <tbody><tr> <td><b>Column- I</b><br><b>Physical quantity</b></td> <td><b>Column-II</b><br><b>MKS system</b></td> <td><b>Column-III<br></b><b>CGS system</b></td> <td><b>Column- IV</b><br><b>FPS system</b></td> </tr> <tr> <td>(A) length</td> <td>(a) newton</td> <td>(p) erg</td> <td>(1) poundal</td> </tr> <tr> <td>(B) mass</td> <td>(b) m</td> <td>(q) dyne</td> <td>(2) ft-poundal</td> </tr> <tr> <td>(C) force</td> <td>(c) kg</td> <td>(r) erg/s</td> <td>(3) ft</td> </tr> <tr> <td>(D) power</td> <td>(d) joule</td> <td>(s) g</td> <td>(4) lb</td> </tr> <tr> <td>(E) work</td> <td>(e) watt</td> <td>(t) cm</td> <td>(5) ft-poundal/s</td> </tr></tbody></table>
Question 16 :
The length $ \ell $, breadth b and thickness t of a block of wood were measured with the help of a measuring scale. The results with permissible errors are $ \ell $ = 15.12 $ \pm $ 0.01 cm, t = 5.28 $ \pm $ 0.01 cm. $ b $ = 10.15 $ \pm $ 0.01 cm. The percentage error in volume upto proper significant figures is :
Question 17 :
A student measures the time period of $100$ oscillations of a simple pendulum four times. The data set is $90\ s, 91\ s, 95\ s$ and $92\ s$. If the minimum division in the measuring clock is $1\ s$, then the reported mean time should be:
Question 18 :
Using the expression $2d sin\theta=\lambda$, one calculates the values of '$d$' by measuring the corresponding angles $\theta$ in the range $0^o$ to $90^o$. The wavelength $\lambda$ is exactly known, and the error in $\theta$ is constant for all values of $\theta$. As $\theta$ increases from $0^o$:
Question 19 :
The relative uncertainty in the period of a satellite orbiting around then earth is $10^{-2}$. If the relative uncertainty in the radius of the orbit is negligible, the relative uncertainty in the mass of the earth is:
Question 20 :
<p>A force $\overrightarrow {\rm{F}} $ is applied on a square plate of length<b> L. </b>If the percentage error in the determination of<b> L</b> is <b>3%</b> and in<b> F</b> is <b>4%,</b> the permissible error in the calculation of pressure is.</p>
Question 21 :
Calculate focal length of a spherical mirror from the following observations.Object distance, u=(50.1±0.5)u=(50.1±0.5) cm<br/>Image distance, v=(20.1±0.2) cm
Question 22 :
$16\ g$ of oxygen and $3\ g$ of hydrogen are mixed and kept at $760\ mm$ pressure and $0^{\circ}C$. The total volume occupied by the mixture will be nearly.
Question 23 :
The length and breadth of a metal sheet are $3.124 \ m$ and $3.002\ m$ respectively. The area of this sheet upto four correct significant figure is :
Question 24 :
The dimension of $\dfrac{B^2}{2 \mu_0}$, where B is magnetic field and $\mu_0$ is the magnetic permeability of vacuum, is:
Question 26 :
Two plates have lengths measured as $(1.9 \pm  0.3)m$ and $(3.5 \pm0.2)m$. Calculate their combined length with error limits.
Question 27 :
A gaseous mixture of ethene, ethane and methane having total volume $150\ ml$ is subjected to combustion in excess of oxygen. If percentage of methane in the original mixture is $20\%$ then calculate volume (in ml) of $C{ O }_{ 2 }\left( g \right)$ which will be obtained at same temperature and pressure.
Question 28 :
Stoke's law states that the viscous drag force $F$ experience by a sphere of radius a, moving with a speed v through a fluid with coefficient of viscosity $\eta$, is given by $F = 6\pi \eta av$<br>If this fluid is flowing through a cylindrical pipe of radius $r$, length $l$ and a pressure difference of $P$ across its two ends, then the volume of water $V$ which flows through the pipe in time $t$ can be written as<br>$\dfrac {v}{t} = k\left (\dfrac {p}{l}\right )^{a}\eta^{b}r^{c}$<br>Where $k$ is a dimensionless constant. Correct values of $a, b$ and $c$ are.<br>
Question 29 :
An experiment measures quantities a, b, c and x is calculated from $x = ab^2/c^3$. If the maximum percentage error in a, b and c are 1%, 3% and 2% respectively, the maximum percentage error in x will be
Question 30 :
Ethanol, ${ C }_{ 2 }{ H }_{ 5 }OH$, is the substance commonly called alcohol, The density of liquid alcohol is $0.7893\ g/ml$ at 293 k. If 1.2 mole of ethanol are needed for a particular experiment, what volume of ethanol should be measured out?
Question 31 :
<br>Pressure depends on distance as, $\displaystyle \mathrm{p}=\frac{\alpha}{\beta}\exp(-\frac{\alpha \mathrm{z}}{\mathrm{k}\theta})$, where $\alpha,\ \beta$ are constants, $\mathrm{z}$ is distance, $\mathrm{k}$ is Boltzman's constant and $\theta$ is temperature. The dimension of $\beta$ are :<br>
Question 32 :
Consider a vessel filled with carbodioxide at $27^{\circ}C$ and $5$ atmospheric pressure. A part of the gas is removed at $27^{\circ}C$ and it fills a $3L$ container at $1\ atm$ and the pressure drops to $3.5\ atm$ in the vessel. The volume of the vessel is:
Question 33 :
Using the expression $ 2\mathrm{d}\sin\theta=\lambda$, one calculates the values of $\mathrm{d}$ by measuring the corresponding angles $\theta$ in the range $\theta$ to $90^{\mathrm{o}}$. The wavelength $\lambda$ is exactly known and the error in $\theta$ is constant for all values of $\theta$. As $\theta$ increases from $0^{\mathrm{o}}$,<br/>
Question 34 :
The distance s travelled by a particle in time t is $s=ut-\displaystyle\frac{1}{2}gt^2$. The initial velocity of the particle was measured to be $u=1.11\pm 0.01$ m/s and the time interval of the experimental was $t=1.01\pm 0.1s$. The acceleration was taken to be $g=9.8\pm 0.1m/s^2$. With these measurements, the student estimates the total distance travelled. How should the student report the result?
Question 35 :
Force $F$ is given in terms of time $t$ and distance $x$ by $F = A\ sin C t + B\ cos D x$ .Then the dimensions of $\displaystyle \frac{A}{B}$ and $\displaystyle \frac{C}{D}$ are given by :
Question 36 :
$ S_{2}O_{3}^{2-}$ ion is oxidized by $ S_{2}O_{8}^{2-}$ ion, the products are $ S_{4}O_{6}^{2-}$ and $ SO_{4}^{-2}$ ions. What volume of 0.25M thiosulphate solution would be needed to reduce 1 g of $ K_{2}S_{2}O_{8}?$ (K = 39)
Question 40 :
In the relation, $\displaystyle P=\frac{\alpha }{\beta }e^{{\alpha z}/{k\theta }}$ $P$ is pressure, $Z$ is distance, $K$ is Boltzmann constant and $\displaystyle \theta $ is the temperature. The dimensions of $\displaystyle \beta $ will be 
Question 41 :
A quantity $f$ is given by $f=\sqrt{\dfrac{hc^5}{G}}$ where c is speed of light, G universal gravitational constant and h is the Planck's constant. Dimension of $f$ is that of?
Question 42 :
The mass of a body is 10.000 gm and its volume is $10.00$ $\mathrm{cm}^{3}$. If the measured values are expressed upto correct significant figures, then the maximum error in the measurement of density is :<br/>
Question 43 :
In particular system of unit, if the unit of mass becomes twice and that of time becomes half, then $8$ Joules will be written as _______ units of work
Question 44 :
With due regard to significant figures, add the following:<br>a. 953 and 0.324<br>b. 953 and 0.625<br>c. 953.0 and 0.324<br>d. 953.0 and 0.374
Question 45 :
The sides of a rectangle are $7.01\ m$ and $12\ m$. Taking the significant figures into account, the area of the rectangle is
Question 46 :
A physical quantity $A$ is related to four observable $a, b, c$ and $d$ as follows, $A = \dfrac {a^{2}b^{3}}{c\sqrt {d}}$, the percentage errors of measurement in $a, b, c$ and $d$ are $1$%, $3$%, $2$% and $2$% respectively. What is the percentage error in the quantity $A$?
Question 47 :
A physical quantity $X$ is given by $X = \dfrac {2k^{3}l^{2}}{m\sqrt {n}}$<br>The percentage error in the measurements of $k, l, m$ and $n$ are $1$%, $2$%, $3$% and $4$% respectively. The value of $X$ is uncertain by
Question 48 :
The refractive index ($\mu$) of glass is found to have the values $1.49, 1.50, 1.52, 1.54\ and\ 1.48$. Calculate percentage error.