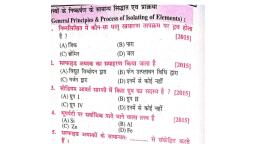
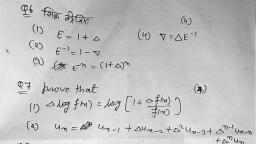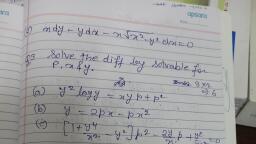Question 1 :
The ratio of mole fraction of a solute and a solvent in a binary solution is:
Question 2 :
A 1.50 g sample of an ore containing silver was dissolved,and all the $ Ag^{+} $ was converted to 0.125 g $ Ag_{2}S $. What was the percentage of silver in the ore?
Question 7 :
A solution of known normality is diluted to two times. Which of the following changes during dilution?
Question 8 :
Number of mole of 1 <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fe38fdd8313cc8726aac"> gas at NTP are:
Question 10 :
Which one of the following set of units represents the smallest and largest amount of energy respectively?
Question 11 :
If $0.01$ mole of solute is present in $500\ ml $ of solution, its molarity is:
Question 12 :
Approximate atomic weight of an element is 26.89 . If its equivalent weight is 8.9 , the exact atomic weight of element would be :- -
Question 13 :
Stoichiometric ratio of sodium dihydrogen orthophosphate and sodium hydrogen orthophosphate required for synthesis of <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fed1a3d2442ab82937d8"> is
Question 14 :
Classify which of the following pair is a compound and a mixture?
Question 15 :
A student performs a titration with different burettes and finds titre values of 25.2 mL, 25.25 mL, and 25.0mL. The number of significant figures in the average titre value is
Question 16 :
How many moles of sulfate ions are in $200 mL$ of a $2 M$ sodium sulfate solution?
Question 17 :
The weight of ${ H }_{ 2 }{ C }_{ 2 }{ O }_{ 4 }.2{ H }_{ 2 }O$ required to prepare 500 ml of 0.2 N solution is:
Question 18 :
0.52 g of dibasic acid required 100 mL of 0.1 <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fccdfdd8313cc8726705"> NaOH for complete neutralization. The equivalent weight of acid is:
Question 19 :
The formula which represents the simple ratio of atoms in a compound is called:
Question 21 :
Equal volumes of $0.1\ M$ $AgN{O}_{3}$ and $0.2\ M$ $NaCl$ are mixed. The concentration of $N{O}_{3}^{-}$ ion in the mixture will be :
Question 22 :
What is the sum of the mole fractions of all the components in a three component system?
Question 23 :
An aqueous solution of glucose is 10% in strength. The volume in which 1 gram mole of it is dissolved will be:<br/>
Question 24 :
What is the molarity of $H_{2}SO_{4}$ solution present initially in the container?
Question 25 :
X gram of pure $As_2S_3$ is completely oxidised to respective highesst oxidation states by 50 ml of 0.1 M hot acidified $KMnO_4$ then X, mass of $As_2S$ taken is :(Molar mass of $As_2S=246$)
Question 26 :
A mixture of $Na_2C_2O_4$ ($A$) and $KH_2C_2O_4.2H_2O$ ($B$) required equal volumes of $0.1$ M $KMnO_4$ and $0.1$ M $NaOH$ separately. Molar ratio of $A$ and $B$ in the mixture is :
Question 28 :
What is the mole fraction of the solute in a $1.00$ m aqueous solution?
Question 29 :
From the following data of $\Delta H$, of the following reactions,<div><br/>$C(s)+\dfrac{1}{2}O_2(g)\rightarrow CO(g)AH=-110$ kJ<br/>$C(s)+H_2O(g)\rightarrow CO(g)+H_2(g)\Delta H=132$ kJ</div><div><br/>What is the mole composition of the mixture of steam and oxygen on being passed over a coke at $1273$ K, keeping the temperature constant?<br/></div>
Question 30 :
Two oleum samples $A$ and $B$ have labeling $109%$ and $118%$ respectively. $100\ g$ of $A$ is diluted to an a $1\ L$ solution and $100\ g$ of $B$ is diluted to a $1000\ mL$ solution. If both these solutions are mixed, then normality of $H_{2}SO_{4}$ (approx.) in final solution is: