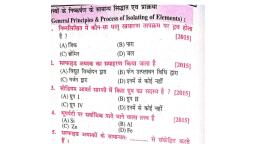
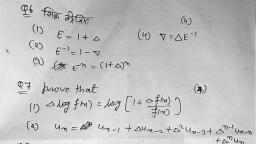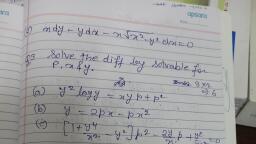Question 1 :
The number of moles of solute present in 2.0 litre of 0.5 M NaOH solution is:
Question 2 :
Stoichiometric ratio of sodium dihydrogen orthophosphate and sodium hydrogen orthophosphate required for synthesis of <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fed1a3d2442ab82937d8"> is
Question 4 :
The number of moles of water in 488 g <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fdb5a3d2442ab829353d"> are:
Question 5 :
The least count of an instrument is 0.01 cm. Taking all precautions, the most possible error in the measurement can be
Question 6 :
Semimolar solution contains how many moles of solute in 1L of solution?
Question 7 :
The number of mole present in 2 litre of 0.5 <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fcdcfdd8313cc872674e"> NaOH is:
Question 8 :
A student performs a titration with different burettes and finds titre values of 25.2 mL, 25.25 mL, and 25.0mL. The number of significant figures in the average titre value is
Question 9 :
A solution of known normality is diluted to two times. Which of the following changes during dilution?
Question 10 :
Classify which of the following pair is a compound and a mixture?
Question 11 :
The formula which represents the simple ratio of atoms in a compound is called:
Question 12 :
The weight of ${ H }_{ 2 }{ C }_{ 2 }{ O }_{ 4 }.2{ H }_{ 2 }O$ required to prepare 500 ml of 0.2 N solution is:
Question 13 :
The weight, in grams, of KCl (Mol.wt. = 74.5) in 100ml of a 0.1M KCl solution is:
Question 15 :
The ratio of mole fraction of a solute and a solvent in a binary solution is:
Question 16 :
X gram of pure $As_2S_3$ is completely oxidised to respective highesst oxidation states by 50 ml of 0.1 M hot acidified $KMnO_4$ then X, mass of $As_2S$ taken is :(Molar mass of $As_2S=246$)
Question 17 :
When 180 grams of glucose is subjected to combustion, the volume of $CO_2$ liberated at STP is
Question 18 :
What is the concentration in molarity of lithium hydroxide prepared by dissolving $48.0$ grams of solid in sufficient water of make $250\ mL$ of solution?
Question 19 :
For a solution of volatile liquids the partial vapour pressure of each component in solution is directly proportional to
Question 20 :
$NaClO$ solution reacts with $H_{2} S_{4}O$. Solution of $NaClO$ used in the reaction contained 15g of NaClO per litre. The normality of the solution would be:
Question 21 :
Potassium permanganate acts as an oxidising agent in acidic, alkaline as well as neutral media. Which among the following statements is incorrect?
Question 23 :
The volume of $0.05 \,M H_{2}SO_{4}$ required to neutralise $80 \,ml $ of $0.13 \,N \,NaOH$ will be
Question 24 :
$5.5\ mg$ of nitrogen gas dissolves in $180\ g$ of water at $273\ K$ and one atm pressure due to nitrogen gas. The mole fraction of nitrogen in $180\ g$ of water at $5\ atm$ nitrogen pressure is approximately :
Question 25 :
100 ml of 10V $H_2O_2$ solution is heated. The evolved gas is completely reacted with Ca to form CaO. The aqueous solution of Cao is neutralised by 50 ml of $H_2SO_4$ solution. The molarity of $H_2SO4$ is: