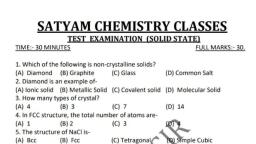
Question 2 :
$4.6$ $cm^3$ of methyl alcohol is dissolved in $25.2$ g of water. Calculate mole fraction of methyl alcohol.
Question 3 :
When $5.0$ gram of $BaCl_2$ is dissolved in water to have $10^6$ gram of solution. The concentration of a solution is:
Question 4 :
The sum of mole fraction of all components of a solution is unity.<br/>
Question 6 :
The mole fraction of water in 20% (wt. / wt.) aqueous solution of $H_2O_2$ is:
Question 7 :
The density of a $10.0\%$ by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.<br/>
Question 8 :
When $1.04 g$ of $BaCl_2$ is present in $105g$ of solution the concentration of solution is:
Question 9 :
State true or false.<br/>$1$ ppm is equal to $1$ mg per kg.
Question 10 :
Assertion: The percentage weight of a compound $A$ in a solution is given by,<br> $\; \; \; \; $ % $\displaystyle of \: A = \frac{Mass \:A}{Total \: mass \: of \: solution}\times 100$<br>$Because$
Reason: The mole fraction of a component $A$ is given by,<br> $\; \; \; \; $ $\displaystyle Mole \: fraction \: of \: A =\frac{Number of \: moles \: of \: A}{ Total \: number\: of \: moles \: of \: all \: components}$
Question 12 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><p class="wysiwyg-text-align-left">Cathodic standard reduction potential minus anodic standard reduction potential is equal to:</p>
Question 14 :
Which of the following will increase the voltage of the cell? <br/><br/>$Sn(s) + 2 \text{Ag}^+ (aq) \rightarrow 2Ag(s) + Sn^{2+}$
Question 15 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><p class="wysiwyg-text-align-left">Which is correct about the reaction between $H_{2}O$ and $O_{2}$ ?</p>
Question 17 :
Which of the following is an example of metallic crystal solid?
Question 18 :
Statement: The melting point of all substances decreases with pressure increase.<br/><br/>State whether the given statement is true or false.
Question 19 :
Which type of bonding is responsible for the ability of some solid materials to conduct electricity?
Question 21 :
Some of the following properties are important in determining whether an element has metallic properties.<br/>I : atomic number<br/>II : atomic weight<br/>III: number of valence electrons<br/>IV: number of vacant atomic orbitals<br/>V: total number of electronic shells in the atom<br/>Select correct properties from the codes given below:
Question 22 :
Solid $X$ is a very hard solid which is electrical insulator in solid as well as in molten state and has extremely high melting point. What type of solid is it?
Question 26 :
Borax is used as cleansing agent because on dissolving in water it gives ________________.
Question 33 :
Which of the following halide of nitrogen is not explosive in nature?
Question 36 :
<span class="wysiwyg-font-size-small"><span class="wysiwyg-font-size-small"><p class="wysiwyg-text-align-left">An element M has the electron configuration $[Ar]3d^{5}4s^{2}$. So, which of the following oxide cannot exist for element M?</p>
Question 39 :
The transitional metal which form green compound in +3 oxidation state and yellow-orange compound in +6 oxidation state is
Question 40 :
Amongst the following ions, which is considered as most stable in ${ M }^{ 2+ }$ state?
Question 43 :
Which major product is formed in the free-radical bromination of methane by limited supply of bromine?
Question 44 :
Statement - 1 : $CH_3 - CH_2 - Cl + NaI \overset{Acetone}{\rightarrow}$ $CH_3 - CH_2 - I + NaCl $<br/>Statement - 2 : Acetone is polar - protic solvent and solubility order of sodium halides decreases dramatically in order $NaI > NaBr > NaCl$. The last being virtually insoluble in the solvent and a $1^0$ and $2^0$ chloro alkane in acetone is completely driven to the side of Iodoalkane by the precipitation reaction.
Question 45 :
Among the isomers of $C_6H_14$ , which will give maximum number of mono-chloro-derivatives ?
Question 46 :
Among the three isomers of the nitrophenol, the one that is least soluble in water is:
Question 47 :
Which of the following alcohols is the least soluble in water?
Question 48 :
A primary amine can be converted to an alchohol by the action of