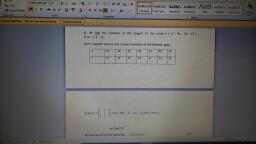
Question 3 :
At the boiling point of water the saturated vapour pressure will be (in mm of Hg)
Question 6 :
Which of the following is not a state function?<br/><b>a.</b> $U + PV$      <b>b.</b> $q + w$     <b>c.</b> $\cfrac { q_ {rev} }{ T }$       <b>d.</b> $q$<br/>
Question 7 :
The following two equilibria exist simultaneously in a closed vessel :<br/>$PCI_5(g) \rightleftharpoons PCI_3(g) + Cl_2(g)$<br/>$COCI_2(g) \rightleftharpoons CO (g) + CI_2 (g)$<br/>If some CO is added into the vessel, then after the equlibrium is attained again, concertration of ?
Question 11 :
Fill in the blank. <br/>Thermodynamics is the branch of science concerned with ____ and ________ and their relation to energy and work. <br/>
Question 15 :
When you combust $100.0\ g$ of propane at $500.K$ and $1.00\ atm$ in a closed container, you expected to collect $279\ L$ of carbon dioxide. Instead, when you collect the gas, it measures $651\ L$ in total.<br>Why have you collected more than your predicted, theoretical yield?
Question 18 :
Which of the following is not a general characteristic of equilibria involving physical processes?
Question 20 :
Properties of substances like pressure, temperature and density, in thermodynamic coordinates are
Question 22 :
An isolated system is one which neither shows an exchange of ____nor ____with surroundings.
Question 27 :
Temperatures of two hot bodies $B_{1}$ and $B_{2}$ are $100^{\circ}C$ and $80^{\circ}C$ respectively. The temperature of surrounding is $40^{\circ}C$. At $t = 0$, the ratio of rates of cooling of the two bodies (liquid) $R_{1} : R_{2}$ will be:
Question 29 :
Which one of the following systems is an example of a closed system?
Question 30 :
Warming ammonium chloride with sodium hydroxide in a test tube is an example of:
Question 31 :
For a reaction to be spontaneous in neither direction, which of the following is/are correct regarding the closed system?<br>(1) ${ \left( \Delta { G } \right) }_{ T,P }=0$<br>(2)${ \left( \Delta { G } \right) }_{ T,P }< 0$<br>(3) ${ \left( \Delta { G } \right) }_{ U,V }=0$<br>(4) ${ \left( \Delta { G } \right) }_{ U,V }>0$<br>Codes:
Question 32 :
A system where there is exchange of energy but not of mass is called _________ system.
Question 33 :
${ N }_{ 2 }(g)+3{ H }_{ 2 }(g)\rightleftharpoons 2N{ H }_{ 3 }(g)$<br>$\Delta { H }^{ o }=-18\quad kJ$<br>According to Le-Chateliers principal the favourable condition for good yield of $NH_{3}$
Question 34 :
$C_v$ for helium gas $(He)$ is $(in J \ mol^{-1} \ K^{-1})$
Question 35 :
When 1 mol gas is heated at constant volume temp. is raised from 298 to 308 K. Heat supplied to the gas is 500 J. Then which statement is correct?
Question 36 :
The temperature at the bottom of a high water fall is higher than that at the top because
Question 39 :
Assertion: Thermodynamics process in nature are irreversible.
Reason: Dissipative effects cannot be eliminated
Question 40 :
Magnitude of Seebeck emf between the junctions does not depend on
Question 42 :
Which of the following property is not a thermodynamic property of the system?
Question 43 :
A reaction that occurs only when heat is added is best described as:
Question 44 :
Thermodynamics mainly deals with<br>Note: Two or more options may be correct.
Question 46 :
For the gas phase exothermic reaction, $A_2+B_2 \rightleftharpoons C_2$, carried out in a closed vessel, the equilibrium moles of $A_2$, can be increased by : 
Question 47 :
When the system is said to be in a state of thermodynamic equilibrium?
Question 48 :
If there were no atmosphere, the average temperature on earth surface would be<br>
Question 50 :
Assertion: The presence of reactants in the closed vessel made of conducting material is an example of a closed system.
Reason: In a closed system, there is no exchange of matter but exchange of energy is possible between the system and the surroundings.
Question 51 :
Two systems are in ____________ when their chemical potentials are the same.<br>
Question 52 :
A system in thermodynamic equilibrium will have the maximum value for : <br/>
Question 55 :
Which of the following are true for all the systems ?
Question 56 :
A system which cannot exchange matter and energy with the surroundings called isolated.<br/>
Question 61 :
<ul><p class="wysiwyg-text-align-justify">Consider the following reactions :<br>$C(s) + {O_2}(g) \to C{O_2}(g) + xkJ$<br>$CO(g) + \frac{1}{2}{O_2}(g) \to C{O_2}(g) + ykJ$<br>The heat of formation of CO(g) is :</p></ul>
Question 63 :
1 : $\Delta U$ is state function.2: Heat and work are not state functions.<br/>
Question 64 :
Assertion: Internal energy change in a cyclic process is zero.
Reason: Internal energy is a state function.
Question 66 :
A system is said to be in thermodynamic equilibrium with surrounding if__________________
Question 68 :
An ideal gas is allowed to expand both reversibly and irreversibly in an isolated system. If $T_i$ is the initial temperature and $T_f$ is the final temperature , which of the following is correct?
Question 69 :
When a liquid in a heat insulated closed vessel is stirred using a peddle then the temperature of the liquid:
Question 70 :
 At certain temperature , the H$^{+}$ ion concentration of water is 4$\times 10^{-7}$ M then the value of  K$_{w}$at the same temperature is :
Question 71 :
Assertion: There cannot be chemical equilibrium in an open system.
Reason: There is no fixed mass in an open system.
Question 75 :
Under which of the following conditions is the relation,<br/>$\triangle H=\triangle U+P\triangle V$ valid for a closed system?
Question 76 :
Select the incorrect set of elements:<br/>I. Work done by the surrounding in case of infinite stage expansion is more than single expansion<br/>II. Ineversible work is always greater than reversible work<br/>III. On an ideal gas in case of single stage expansion and compression system as well as surrounding are restored back to their original states<br/>IV. If gas is in thermodynamic equilibrium is taken from state $A$ to state $B$, by four successive single stage expansions. Then we can plot $4$ points on the P.V indicator diagram
Question 77 :
Assertion: We feel cold on touching the ice.
Reason: Ice is a solid form of water.
Question 78 :
When a volatile liquid is introduced into an evacuated closed vessel at a particular temperature, both evaporation and condensation take place simultaneously. The system reaches equilibrium state when:
Question 79 :
Assertion: Work and internal energy are not state functions.
Reason: The sum of $\displaystyle q+w$ is state function.
Question 80 :
Choose the correct answer. A thermodynamic state function is a quantity : 
Question 81 :
System in which there is no exchange of matter, work or energy from surroundings is:
Question 82 :
Which of the following state function not zero at standard state?<br/>
Question 84 :
For a first order reaction rate constant is $1 \times {10^{ - 5}}{\sec ^{ - 1}}$ having ${E_a} = 1800\ kJ/mol$ . Then the value of $\ell nA$ at$T = 600\ K$ is:-
Question 85 :
"Heat cannot by itself flow from a body at lower temperature to a body at higher temperature" is a statement of the consequence of
Question 86 :
In a laboratory, liquid in a thermally insulated container is stirred for one hour by a mechanical linkage to a stirrer, for this process:
Question 87 :
Water of mass $m_2$ = 1 kg is contained in a copper calorimeter of mass $m_1$  = 1 kg. Their common temperature t = $10^{0}C$. Now a piece of ice of mass $m_3$  = 2 kg and temperature is $-11^{0}C$ dropped into the calorimeter. Neglecting any heat loss, the final temperature of system is. [specific heat of copper = 0.1 Kcal/ kg$^{0}C$, specific heat of water = 1 Kcal/kg$^{0}C$, specific heat of ice = 0.5 Kcal/kg$^{0}C$, latent heat of fusion of ice = 78.7 Kcal/kg]
Question 88 :
At $227^0C,\,60\%$ of 2 moles of $PCl_5$ gets dissociated in a two-litre. the value of $K_p$ will be
Question 89 :
Two closed vessel $A$ and $B$ of equal volume of $8.21L$ are connected by a narrow tube of negligible volume with open valve. The left hand side container id found to contain $3\ mole \, CO_2$ and $2\ mole$ of $He$ at $400K$. What is the partial pressure of $He$ in vessel $B$ at $500K$?
Question 90 :
In an evacuated closed isolated chamber at ${250}^{o}C$. $.02$ mole $P{Cl}_{5}$ and $.01$ mole ${Cl}_{2}$ are mixed $(P{Cl}_{5}\rightleftharpoons P{Cl}_{3}+{Cl}_{2})$. At equilibrium density of mixture was $2.49g/L$ and pressure was $1$ atm. The number of total moles at equilibrium will be approximately:
Question 91 :
<p class="wysiwyg-text-align-left">The internal energy change when a system goes from state $A$ to $B$ is $40 \ kJmole^{-1}$. If the system goes from $A$ to $B$ by a reversible path and returns to state $A$ by an irreversible path, what would be the net change in internal energy?</p>
Question 92 :
A child bought a balloon which became very small in size the next day. Which is correct statement about balloon?
Question 95 :
An insulated container of gas has two chambers separated by an insulating partition. One of the chambers has volume $V_1$ and contains ideal gas at pressure $P_1$ and temperature $T_1$. <br/>The other chamber has volume $V_2$ and contain same ideal gas at pressure $P_2$ and temperature $T_2$. If the partition is removed without doing any work on the gas, the final equilibrium temperature of the gas in the container will be:
Question 97 :
Calculate the amount of ice that will separate out on cooling a solution containing $50$ g of ethylene glycol in $200$g of water to $-9.3°C$.Molal depression constant for water is $1.86$ K kg/mol
Question 98 :
Use the given standard enthalpies of formation to determine the heat of reaction of the following reactions:<br>$TiCl_{4}(g) + 2H_{2}O(g) \rightarrow TiO_{2}(g) + 4HCl(G)$<br>$\triangle H_{f}^{\circ}TiCl_{4}(g) = -736.2\ kJ/mole\ \triangle H^{\circ}_{f} TiO_{2}(g) = -944.7\ kJ/mole$<br>$\triangle H_{f}^{\circ} H_{2}O(g) = -241.8\ kJ/mole\ \triangle H^{\circ}_{f} HCl(g) = -92.3\ kJ/mole$.
Question 99 :
<p class="wysiwyg-text-align-left">Assertion (A): A system is said to be closed if it can exchange energy but not matter. </p><p class="wysiwyg-text-align-left">Reason (R): Coffee in a stoppered thermos flask is an example of closed system.<br/></p>