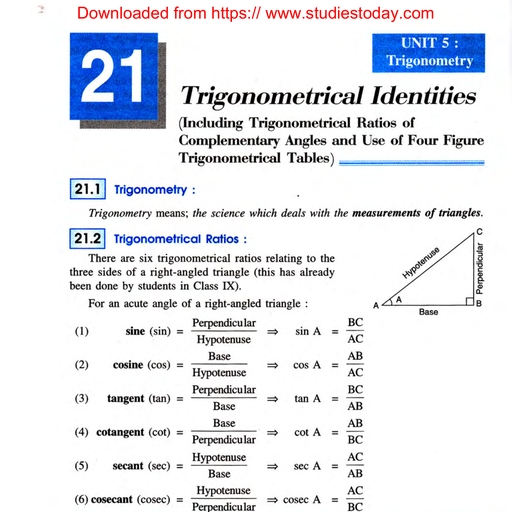
Question 3 :
$1.0 \ L$ solution is prepared by mixing $61 \ gm$ benzonic acid $(pK_a = 4.2)$ with $72 \ gm$ of sodium benzoate and then $\text{300 mL 1.0 M HBr}$ solution was added. the $pH$ of final solution is:-
Question 4 :
The hydrogen ion concentration of the oceans is about $2\times10^{-9}\space M$. What is the $pH$?
Question 5 :
If $H^{+}$ion concentration of a solution is increasedby $10$ times, its $pH$ will :<br>
Question 7 :
Effervescence is seen when a bottle of soda water is opened.
Question 8 :
The solution of bleaching powder in water is always a clear solution.<br/>
Question 11 :
Water of crystallization is the fixed number of water molecules chemically attached to a unit of a salt in its crystalline form.<br/>
Question 16 :
$pK_{a}$ of acetic acid is <b>4.741</b>. The concentration of $CH_{3}COONa$ is <b>0.01</b> M. The pH of $CH_{3}COONa$ is?
Question 18 :
When the soil is too basic, plants do not grow well in it. To improve its quality what must be added to the soil?
Question 19 :
In which of the following reactions does conc.${ H }_{ 2 }S{ O }_{ 4 }$ act as an oxidising agent?
Question 20 :
Which part of tooth, made up of calcium phosphate, is the hardest substance in the body?
Question 22 :
Which colour appears when few drops of phenolphthalein put into test tube contains lime water?
Question 23 :
Four solutions A, B, C and D when tested with universal indicator showed pH as $4, 11, 7$ and $9$ respectively. Which one of the four solutions is neutral?<br/>
Question 25 :
The acid present in the body of red ant is ________.<br/>
Question 26 :
Which of the following methods is adopted for preparing dilute solution of ${ H }_{ 2 }S{ O }_{ 4 }$?
Question 27 :
$p{ K }_{ a }$ values of four acids are given below at ${ 25 }^{ o }C$. The strongest acid is:
Question 29 :
The chloride salt of a certain weak monoacidic organic base is hydrolysed to an extent of $3$% in its $0.1M$ solution at ${25}^{o}C$. Given that the ionic product of water is ${10}^{-14}$ at this temperature, what is the dissociation constant of the base?
Question 30 :
When $NH_{3}(0.1\ M) 50\ ml$ mix with $HCl (0.1\ M)\ 10\ ml$ then what is $pH$ of resultant solution $(Pk_{b} = 4.75)$.
Question 33 :
Aquaregia is prepared by mixing conc. $HCl$ and $conc. HNO_3$ in the ratio ________ 
Question 36 :
Calculate the pH of a solution which is 0.1 M in HA and 0.5M in NaA. Ka for HA is $1.8\times { 10 }^{ -6 }$?
Question 37 :
Statement I: A basic solution has more hydrogen ions than an acidic solution.<br/>Statement II: pH is defined as the -log $[H^+]$.<br/>
Question 38 :
If tartaric acid is not added in baking powder, the cake will taste bitter due to te presence of -