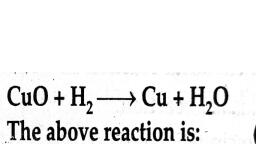Question 2 :
$1.0 \ L$ solution is prepared by mixing $61 \ gm$ benzonic acid $(pK_a = 4.2)$ with $72 \ gm$ of sodium benzoate and then $\text{300 mL 1.0 M HBr}$ solution was added. the $pH$ of final solution is:-
Question 5 :
Four solutions A, B, C and D when tested with universal indicator showed pH as $4, 11, 7$ and $9$ respectively. Which one of the four solutions is neutral?<br/>
Question 8 :
Which of the following methods is adopted for preparing dilute solution of ${ H }_{ 2 }S{ O }_{ 4 }$?
Question 9 :
Effervescence is seen when a bottle of soda water is opened.
Question 10 :
$p{ K }_{ a }$ values of four acids are given below at ${ 25 }^{ o }C$. The strongest acid is:
Question 11 :
If tartaric acid is not added in baking powder, the cake will taste bitter due to te presence of -
Question 13 :
In which of the following reactions does conc.${ H }_{ 2 }S{ O }_{ 4 }$ act as an oxidising agent?
Question 15 :
Aquaregia is prepared by mixing conc. $HCl$ and $conc. HNO_3$ in the ratio ________ 
Question 16 :
Which part of tooth, made up of calcium phosphate, is the hardest substance in the body?
Question 20 :
When $NH_{3}(0.1\ M) 50\ ml$ mix with $HCl (0.1\ M)\ 10\ ml$ then what is $pH$ of resultant solution $(Pk_{b} = 4.75)$.
Question 22 :
Statement I: A basic solution has more hydrogen ions than an acidic solution.<br/>Statement II: pH is defined as the -log $[H^+]$.<br/>
Question 24 :
The chloride salt of a certain weak monoacidic organic base is hydrolysed to an extent of $3$% in its $0.1M$ solution at ${25}^{o}C$. Given that the ionic product of water is ${10}^{-14}$ at this temperature, what is the dissociation constant of the base?
Question 26 :
The acid present in the body of red ant is ________.<br/>
Question 28 :
The solution of bleaching powder in water is always a clear solution.<br/>
Question 30 :
Calculate the pH of a solution which is 0.1 M in HA and 0.5M in NaA. Ka for HA is $1.8\times { 10 }^{ -6 }$?
Question 33 :
Assertion: pH of a neutral solution is always $7$.
Reason: pH of a solution does not depend upon temperature.
Question 34 :
The hydrogen ion concentration of the oceans is about $2\times10^{-9}\space M$. What is the $pH$?
Question 35 :
Water of crystallization is the fixed number of water molecules chemically attached to a unit of a salt in its crystalline form.<br/>
Question 37 :
$pK_{a}$ of acetic acid is <b>4.741</b>. The concentration of $CH_{3}COONa$ is <b>0.01</b> M. The pH of $CH_{3}COONa$ is?
Question 38 :
If $H^{+}$ion concentration of a solution is increasedby $10$ times, its $pH$ will :<br>
Question 40 :
When the soil is too basic, plants do not grow well in it. To improve its quality what must be added to the soil?
Question 42 :
Which colour appears when few drops of phenolphthalein put into test tube contains lime water?
Question 43 :
Assertion: Sodium bicarbonate is an acidic salt.
Reason: It gives basic solution in water.
Question 44 :
The pH of a ${10}^{-8} M$ solution of ${CH}_{3}COOH$ would be:
Question 46 :
Washing soda on strong heating gives sodium oxide and carbon dioxide.<br/>
Question 49 :
By which we can measure the exact $pH$ of aqueous solution?
Question 50 :
What is the $pH$ of a saturated solution of $X(OH)_3$ will be if its solubility product = $2.7 \times 10^{-43}$?<br/>[Given: log 3 = 0.5, log 2 = 0.3]
Question 51 :
The $pH$of $0.1\ M$ solution of the following salts increases in the order
Question 53 :
What is the nature of solution at ${60}^{o}C$ whose $pH$ is $6.35$?
Question 54 :
Consider the following statement and select correct option :<br>(l) $K_{sp}$ of $Fe(OH)_3$ in aqueous solution is 3.8 x $10^{38}$ at 298 K. The concentration of $Fe^{3+}$ will increase when $[H^+]$ ion concentration decreases.<br>(ll) In a mixture of $NH_4Cl$ and $NH_4OH$ in water, a further amount of $NH_4Cl$ is added. The pH of the mixture will decreases.<br>(lll) An aqueous solution of each of the following salts $(NH_4l, HCOOK)$ will be basic, acidic respectively.
Question 56 :
Which of the following items are not bleached by bleaching powder?
Question 59 :
The gatric juice in our stomach contains enough $HCl$ to make the hydrogen ion concentration about $0.01\ mol .L^{-1}$. The pH of gastric juice is:
Question 63 :
Assertion: pH of 10 M $HCl$ aqueous solution is less than 1.
Reason: pH is negative logarithm of $H^{+}$ concentration.
Question 65 :
The pHof a soft drink is $3.82$. The hydrogen ion concentration wil be: (given antilog $0.18=1.5$)
Question 69 :
The amount of chlorine obtained from a sample of bleaching powder by treatment with excess of dilute acidor $ \displaystyle CO_{2}$ is called available chlorine.
Question 70 :
Calamine is used to reduce the irritating effect of ant bite/sting because it reacts with (X) released due to the bit /sting of ants with (Y) present in calamine. (X) and (Y) respectively are:
Question 71 :
The pH of an $HCl$ solution is $2$. Sufficient water is added to make the pH of new solutions. The hydrogen ion concentration is reduced:
Question 75 :
Copper sulphate pentahydrate is commonly called as_______, whereas ferrous sulphate heptahydrate is commonly called as ________ .
Question 76 :
$K_a$ for $CH_3COOH$ is $1.8\times 10^{-5}$ and $K_b$ for $NH_4OH$ is $1.8 \times 10^{-5}$. The pH of ammonium acetate will be:<br/>
Question 78 :
State whether the given statement is true or false:<br/>Bleaching powder contains about 50 % available chlorine.<br/>
Question 80 :
What is the $pH$ of a solution that contains $0.1\ mol/L$ of ${H}_{3}{O}^{+}$ ions?
Question 81 :
$CaCO_3 \rightarrow CaO +CO_2$ <br> $E + CO_2 \rightarrow F$ <br> $NaCl + F \rightarrow A+ NaHCO_3$The other compound formed with sodium bicarbonate is:<br/>
Question 82 :
The element which is not common between the compounds called baking soda and soda ash is:
Question 83 :
The ionization constant of a monobasic acid is $2.24\times 10^{-2}$. The $pH$ of $0.01\ M$ acid solution is:
Question 84 :
If the pH of pure water is $7$, then the hydrogen ion concentration, $[H^{+}]$ in mol L$^{-1}$ will be:
Question 87 :
Bleaching powder loses ___________ by the action of dilute acids in excess.
Question 89 :
Read the given statements and select the correct option:<br/>Statement 1 :Backing soda does not taste sour.<br/>Statement 2 :Bases are sour in taste while acids are bitter in taste.
Question 90 :
The pH values for three different solutions are $13,\ 11$ and $9$. Arrange these pH values in the decreasing order of hydronium or ${H}^{+}$ ion concentration.
Question 91 :
Which of the following salts does not contain water of crystallisation ?
Question 92 :
${ H }_{ 2 }O+{ H }_{ 3 }{ PO }_{ 4 }\rightleftharpoons { H }_{ 3 }{ O }^{ + }+{ H }_{ 2 }{ PO }_{ 4 }^{ - };  \ \ \ \ p{ K }_{ 1 }=2.15$<br/>${ H }_{ 2 }O+{ H }_{ 2 }{ PO }_{ 4 }^{ - }\rightleftharpoons { H }_{ 3 }{ O }^{ + }+{ H }_{ 2 }{ PO }_{ 4 }^{ 2- };\ \ \ p{ K }_{ 2 }=7.20$<br/>Hence, pH of $0.01M$ $Na{ H }_{ 2 }{ PO }_{ 4 }$ is:
Question 93 :
$NaHCO_{3}$ can be used on its own to make cakes or bread. Baking powder is more commonly used, and contains $NaHCO_{3}$, $Ca(H_{2}PO_{4})_{2}$ and starch. An improved combination baking powder contains about 40% starch, 30% $NaHCO_{3}$ 20% $NaAl(SO_{4})_{2}$ and 10% $Ca(H_{2}PO_{4})_{2}$Answer the following questions based on above study.<br/><br/>In improved baking powder, $NaAl(SO_{4})_{2}$ is used for :
Question 94 :
Which is the correct for the following reaction: <br/>$B\, (OH)_3\, +\, H_2O\, \rightarrow \, [B(OH)_4]^-\, +\, H^+$
Question 96 :
What is the correct increasing order of the $pH$ values of the following solutions with equal concentrations?
Question 98 :
The $pH$of $0.1 M$ solution of the following salts increases in the order
Question 99 :
The third dissociation constant of $H_{3}PO_{4}$ is $1.3\ \times 10^{-12}$. Assume that the hydrolysis proceeds only in the first step, the $pH$ of $0.1\ M\ K_{3}PO_{4}$ solution is:
Question 100 :
The ions responsible for the bleaching action of bleaching powder and the ions responsible for loss of bleaching action of bleaching powder due to the storage for long time respectively are:<br/>
Question 101 :
$NaHCO_{3}$ can be used on its own to make cakes or bread. Baking powder is more commonly used, and contains $NaHCO_{3}$, $Ca(H_{2}PO_{4})_{2}$ and starch. An improved combination baking powder contains about 40% starch, 30% $NaHCO_{3}$ 20% $NaAl(SO_{4})_{2}$ and 10% $Ca(H_{2}PO_{4})_{2}.$ Answer the following questions based on above study.<br/>$Ca(H_{2}PO_{4})_{2}$ in baking powder:
Question 102 :
$\dfrac{N}{10}$ acetic acid was titrated with $\dfrac{N}{10}$ $NaOH$. when 25%, 50% and 75% of titration is over then the $pH$ of the solution will be (respectively): $[K_a = 10^5]$ 
Question 103 :
$1\ cc$ of $0.1N$ $HCl$ is added to $1$ litre solution of sodium chloride. The $pH$ of the resulting solution will be:
Question 104 :
Baking powder used to make a cake is a mixture of starch, $NaHCO_3$ and $Ca(H_2PO_4)_2$. The function of $Ca(H_2PO_4)_2$ is:<br/>
Question 105 :
The indicator constant for an acidic indicator , HIn is $ 5 \times 10^{-6}  M $. this indicator appears only in the colour of acidic from when $ \frac { [In^- ] }{ [HIn]} \le \frac {1}{20} $ and it appears only in the colour of basic form when $ \frac { [HIn]}{[In^-]} \le \frac {1}{40} . $ the pH range of indicator is:
Question 106 :
One mole of ammonia was completely absorbed in one litre solution each of<br/>I. $1\ M\ HCl$<br/>II. $1 \ M\ CH_3COOH$ <br/>III. $1\ M\ H_2SO_4$ at $298$ K<br/>The decreasing order for the $pH$ of the resulting solution is:(Given: $K_b (NH_3) = 4.74$)
Question 107 :
The first and second dissociation constants of an acid $H_{2}A$ are $1.0\times 10^{-5}$ and $5.0\times 10^{-10}$ respectively. The overall dissociation constant of the acid will be:
Question 108 :
The $pH$ of $HCl$ is 1. $1.10\ ml$ of this solution is reacted with $40\ ml$ of $NaOH$ solution whose $pH=12$. The $pH$ of resulting solution is: $(\log(1.2)=0.108)$<br/>
Question 109 :
The more commonly used baking powder contains about 30% $NaHCO_3$, 20% $NaAl(SO_4)_2$, 10% $Ca(H_2PO_4)_2$ and 40% starch. Which of the following statements are correct?<br/>
Question 110 :
Dissociation constants of $CH_3COOH$ and $NH_4OH$ in aqueous solution are $10^{-5}$. If pH of a $CH_3COOH$ solution is $3$. What will be the pH of $NH_4OH$?
Question 111 :
The $pH$ of a buffer solution containing equal molar concentration of a week base and its chloride ($K_{b}$ for weak base $=2\times 10^{-5}$) is :
Question 112 :
Iso-electric point of alanine is (pH = $6$). At which pH, maximum concentration of zwitter ion of alanine will be present?
Question 113 :
$1.0$ ml of a solution of $HCl$ with a $pH$ of $4.0$ is added it to $9.0$ ml of distilled water, the $pH$ of the final solution will be:
Question 114 :
The aqueous solution of which one of the following is basic?
Question 117 :
Number of $OH^-$ in 1 mL solution of pH = 13 is:
Question 118 :
Percentage ionisation of water at certain temperature is $3.6\times { 10 }^{ -7 }$%. Calculate ${K}_{w}$ and $pH$ of water.
Question 119 :
When chlorine is passed over dry slaked lime at room temperature, the main reaction product is:
Question 123 :
The $pH$ at which a 0.01 M ${ Al }^{ +3 }$ solution is 99.99% precipitated is: $\left( { K }_{ sp } \ \ Al\left( OH \right) _{ 3 }=1\times { 10 }^{ -18 }at\quad 25 \right) $
Question 124 :
Substance $X$:<br/>(I) reacts with $H_2S$ to produce white turbidity.<br/>(II) changes light green solution of $FeSO_4$ into yellow colour.<br/>(III) reacts with moisture to give pungent smelling gas.<br/>Hence $X$ is:
Question 125 :
When ethyl alcohol is heated with a paste of bleaching powder, we get a compound in which the function of bleaching powder is: 
Question 126 :
Which one of the following solutions will have the highest pH value?
Question 128 :
To $1.0 L$ solution containing $0.1$ mol each of $NH_3$ and $NH_4Cl, 0.05$ mol NaOH is added. The change in pH will be : $(pK_a$ for $CH_3 COOH=4.74$)
Question 129 :
The $pH$ of $0.1M$ solution of the following salts increases in the order:
Question 130 :
$NaHCO_{3}$ can be used on its own to make cakes or bread. Baking powder is more commonly used, and contains $NaHCO_{3}$, $Ca(H_{2}PO_{4})_{2}$ and starch. An improved combination baking powder contains about 40% starch, 30% $NaHCO_{3}$ 20% $NaAl(SO_{4})_{2}$ and 10% $Ca(H_{2}PO_{4})_{2}.$ Answer the following questions based on above study.<br/>Rise in bread and cakes is due to______ when it decomposes between $50^{o}C$ and $100^{o}C$. 
Question 133 :
The $pH$ of a ${ 10 }^{ -9 }\ M$ solution of $HCl$ in water is :<br>
Question 135 :
A magnesium ion has a net positive charge while the magnesium atom has __________. 
Question 137 :
Which among the following metals is employed to provide cathodic protection to iron?
Question 140 :
The process of protecting iron by coating with zinc is called:
Question 142 :
Which of the following metals exist in their native state in nature?<br/>(i) Ca<br/>(ii) Au<br/>(iii) Zn<br/>(iv) Ag<br/>
Question 146 :
Most of the metals which occurs in native state in nature:
Question 150 :
Assertion: Ionic compounds are bad conductors of electricity.
Reason: In ionic compounds mobile ions exists.
Question 152 :
Which one of the methods given in column- I is applied for the extraction of the metal given in column II:<br/><blockquote><table class="wysiwyg-table"> <tbody><tr> <td><b>Column -I</b></td> <td><b>Column - II</b></td> </tr> <tr> <td>(A) Electrolytic reduction</td> <td>(i) Aluminium</td> </tr> <tr> <td>(B) Reduction with carbon</td> <td>(ii) Zinc</td> </tr> <tr> <td>(C) Reduction with aluminium</td> <td>(iii) Sodium</td> </tr> <tr> <td> </td> <td>(iv) Iron</td> </tr> <tr> <td> </td> <td>(v) Manganese</td> </tr> <tr> <td> </td> <td>(vi) Chromium</td> </tr></tbody></table></blockquote>
Question 153 :
Electrolysis is used in the extraction of metals from their ores.
Question 154 :
An ore contains arsenic and antimony as impurities. How are they removed during the process of roasting?<br/>
Question 157 :
A solid compound "A" consists of simple ions.<br/>Nature of the bond present in A will be:
Question 159 :
When manganese dioxide is heated with aluminium powder, the products obtained are _________ .<br/>
Question 163 :
Coulomb's law is used to calculate the energy of an ion pair.
Question 164 :
Heating of concentrated ore in absence of air, for conversion into oxide ore, is known as ____.<br/>
Question 167 :
The complete loss of an electron of one atom to another atom with the consequent formation of electrostatic charges is said to be
Question 168 :
During the formation of sodium chloride, sodium transfers one electron to chlorine to attain the configuration of which noble gas?
Question 170 :
 Galvanizing is a process of coating iron with a metal to prevent it from rusting. The metal is :
Question 171 :
Galvanisation is a method of protecting iron from rusting, by coating with a thin layer of:<br/>
Question 172 :
Read the following statements and answer as true or false.<br/>Metals occur in nature only as free elements.<br/>
Question 174 :
During electrolytic refining of copper.<br/>(a) Pure copper acts as anode.<br/>(b) Pure copper acts as cathode.<br/>(c) Impure copper acts as anode.<br/>(d) Impure copper acts as cathode.<br/>
Question 176 :
The second most abundant metal present in the crust of the earth is :
Question 177 :
The structure of glycine (amino acid) is $H_{2}N-CH_{2}COO^{-}$ ($z$ witter ion). Select the correct statement:-
Question 178 :
The process to heat the ore in the presence of excess supply of air below its melting point is called as ________.<br/>
Question 180 :
During the formation of ionic bond ________transfer takes place from one atom to the others.
Question 182 :
In case of formation of ionic compounds, the electronegativity difference between them is less than 1.7.<br/>
Question 184 :
Compare NaCl and CsCl with respect to ease of formation and also the strength of the ionic bond.
Question 187 :
The solubility of $KCl$ is relatively more in___________.(where $D$ is dielectric constant)
Question 190 :
In the compound between A and H, what type of bond is formed?
Question 191 :
The reactivity series is a list of metals arranged in the order of their _______ activities.
Question 192 :
Two elements whose electronegatives are 1.2 and 3.0, the bond formed between them would be:
Question 194 :
The ionic bonds $X^{\displaystyle+}Y^{\displaystyle-}$ are formed when<br>(I) electron affinity of $Y$ is high<br>(II) ionization energy of $X$ is low<br>(III) latticeenergy of $XY$ is high<br>(IV)latticeenergy of $XY$ is low
Question 195 :
Which of the following methods is not used to coat metallic elements to prevent corrosion?
Question 196 :
An element X has low ionization energy and another element Y has high electron affinity. The bond formed between them could be:
Question 199 :
The order of reactivity of $K, Mg, Au$ and $Zn$ with water is:
Question 200 :
Roasting of sulphides gives the gas $X$ as a byproduct. This is colourless gas with choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as a reducing agent and its acid has never been isolated. The gas $X$ is:<br/>
Question 203 :
The type of bond between atoms of potassium and chloride in a crystal of potassium chloride is :
Question 204 :
Which of the following statement is correct for the attainment of stable electronic configuration in case of magnesium atom?
Question 206 :
Which one of the following metals do not react with cold as well ashot water?<br>
Question 209 :
Given below are the steps for extraction of copper from its ore. The balance reaction involved in - <br/><br/>Roasting of copper(I) sulphide is:<br/>
Question 210 :
Which pair in the following has maximum and minimum ionic character respectively?<br/>
Question 213 :
The number of electron involved in the bond formation of $N_2$ molecule?
Question 214 :
$Li$, $K$, $Ca$, $Na$, $Mg$, $Al$, $Zn$, $Fe$, $Pb$, $Cu$, $Hg$, $Ag$<br/>$I$. The reaction $3Fe(s)+2Al{({NO}_{3})}_{3}(aq)\rightarrow 2Al(s)+3Fe{({NO}_{3})}_{2}(aq)$ will occur.<br/>$II$. Iron is higher on the activity series than aluminum.
Question 216 :
Which cannot be obtained by electrolysis of the aqueous solution of their salt?<br/>
Question 220 :
Among $Mg,Cu,Fe,Zn$ the metal that does not produce hydrogen gas in reaction with hydrochloric acid is
Question 221 :
Assertion: Ionic compounds are bad conductors of electricity.
Reason: In ionic compounds mobile ions exists.
Question 223 :
Transfer of electrons from one atom to other atom takes place in:<br/>
Question 228 :
What happens when a piece of silver metal is added to copper sulphate solution?<br/>
Question 230 :
Copper can be displaced from aqueous solution of copper sulphate by adding:
Question 231 :
Assertion: Molten potassium chloride is a good electrical conductor.
Reason: the melting process frees the ions in potassium chloride from their fixed positions in the crystal lattice.
Question 232 :
Four metals and their methods of refinement are given.<br/>(i) Ni, Cu, Zr, Ga<br/>(ii) Electrolysis, van Arkel process, zone refining, Mond's process.<br/>Choose the right method for each.<br/>
Question 235 :
Which of the following equilibria would have the lowest value of $K_{p}$ at a common temperature?
Question 236 :
Roasting is carried out to :1) convert sulphide to oxide and sulphate. <br/>2) remove the water of hydration. <br/>3) melt the ore. <br/>4) remove arsenic and sulphur impurities.<br/>Choose the correct options.
Question 237 :
Roasting of copper pyrite ores is for which of the following purpose?
Question 238 :
Which set of compounds in the following pair of ionic compounds has the higher lattice energy?<br/>(I) $KCl$ or $MgO$<br/>(II) $LiF$ or $LiBr$<br/>(III) $MgF_2$ or $NaCl$<br/>
Question 241 :
Which of the following family form negative ions in an ionic bond?
Question 242 :
Which of the following statement is incorrect about the extraction of lead from galena?<br>
Question 244 :
In self reduction process, the species which bring about the reduction is :<br/>
Question 246 :
Which one of the following reactions will occur on heating $AgNO_3$?
Question 247 :
Assertion: Ionic bonds are always stronger than covalent bonds.Reason: They break only when bombarded with electrons.
Question 249 :
Which of the following process is used in the extractive metallurgy of magnesium ?
Question 253 :
Which of the following sets of characteristics leads to the increase in solubility of ionic substances?
Question 254 :
Assertion: In smelting, roasted ore is heated with powdered coke in presence of a flux.
Reason: Oxides are reduced to metals by $C$ or $CO$. Impurities are removed as slag.
Question 255 :
<p>Some of the following metals are obtained by electrolysis of their fused salts.</p><p>$Al, Na, Cu, Ag, Mg, Ca$<br/></p><p>These metals are:</p>
Question 256 :
Which of the following is not a property of an ionic compound?
Question 258 :
Sodium is made by the electrolysis of molten mixture of about $40\% \ NaCl$ and $60\%$ $CaCl_2$ because:
Question 259 :
ZnO in step (ii) can also be dissolved in NaOH forming :
Question 260 :
Mark the correct statements:<br/><br/>(i) mercury can be refined by the process of distillation.<br/><br/>(ii) In poling, the molten impure metal is stirred with green poles of wood.<br/><br/>(iii) In electrolyte refining of metals, impure metal is made as cathode and a thin strip of pure metal is made as anode.
Question 262 :
Select the correct reason for given statement 'Fluoride of $Al$ is ionic while chloride is covalent'.
Question 264 :
Which of the following has a bond formed from the transfer of electrons?
Question 265 :
When metallic copper comes in contact with moisture, a green powdery / pasty coating can be seen over it. This is chemically known as
Question 266 :
Which one of the following metals will not reduce $ H_2 O? $
Question 267 :
Assertion (A): In the electrolysis of aqueous NaCl,Na is preferentially discharged at the mercurycathode forming sodium amalgam.<br>Reason (R) : It is due to the fact that hydrogenhas a high over voltage at mercury cathode.<br>
Question 269 :
$FeS + HCl \rightarrow A + B(g)$<br/><br/>Gas $B$ is passed into aqueous solution of $C$ to form $A$. Select correct statements based on the above facts.
Question 270 :
Assertion: Statement $\displaystyle -$ 1 : The first ionisation energy of N is greater than O.
Reason: Statement $\displaystyle -$ 2 : N atom has half filled p $\displaystyle -$ orbitals.
Question 271 :
The element X reacting with chlorine forms a water soluble compound having high melting point. Element X is:
Question 272 :
Consider the following statements. Roasting is carried out to:<br/>(i) convert sulphide to oxide and sulphate.<br/>(ii) to remove impurities of $CuS$ and $FeS$ present in the ore of tin stone $(SnO_2)$ as $CuSO_4$ and $FeSO_4$.<br/>(iii) melt the ore.<br/>(iv) remove arsenic and sulphur impurities.<br/>Of these statements:<br/>
Question 276 :
Statement 1: Powdered zinc reacts faster with acid than a larger piece of zinc.<br/>Statement 2: Powdered zinc has a greater surface area.
Question 277 :
Among the following pairs of oxides, In which pair both are reduced by carbon?
Question 278 :
In $Fe-$extraction, the roasting is adopted though the ore is no having any sulphide because:
Question 279 :
When a group IIA atom forms an ion, what charge does it have?
Question 282 :
For purification of alumina, the modern processes most useful when (i) The impurity present is lot of iron oxides and (ii) the impurity present is a lot of silica, are
Question 283 :
Amongst $LiCl$, $ BeCl_2,\ MgCl_2 $ and $RbCl$ the compounds with greatest and least ionic character, respectively are:
Question 284 :
Identify the type of reaction which will occur based on the information provided about the reactants:<br>${CH}_{4}(g)+{O}_{2}(g)\rightarrow$
Question 285 :
An oxidizing agent is always takes part in the reduction process while a reducing agent is always take part in the _______ process.
Question 287 :
In $HS^, I^, R-NH_2, NH_3$ order of proton excepting tendency will be:
Question 288 :
The substance that initially involved in a chemical reaction is called a reactant.
Question 290 :
A less reactive metal replaces a more reactive metal from its salt solution in water.<br>
Question 291 :
Three cations $A, B, C$ are allowed to react with ammonium hydroxide solution. $A$ and $B$ produce a dirty green precipitate and a reddish brown precipitate respectively, both of which do not dissolve on further addition of ${NH}_{4}OH$. $C$ gives pale blue colour precipitate with less amount of ammonium hydroxide and on further addition of ${NH}_{4}OH$, precipitate becomes soluble and deep blue colour solution is formed:
Question 293 :
AB + CD $\rightarrow$ AD + BC<br/><br/>The reaction is an example of _________.<br/><br/><br/>
Question 294 :
$KXO_3$(s) well mixed with water, temperature of solution falls. This reaction is:<br/>
Question 296 :
Which of the following is an example of the exothermic reaction ?
Question 298 :
In the reaction, $4Fe+3O_2\rightleftharpoons 4Fe^{3+}+6O^{2-}$ Which of the following statements is incorrect?
Question 299 :
State true or false.<br/>In a chemical equation, the products are written on the left-hand side and the reactants on the right-hand side with an arrow in between.
Question 300 :
Name the type of the following chemical reaction.$2KClO_3 \rightarrow 2KCl +3O_2$
Question 301 :
$FeCl_3 + 3NaOH \rightarrow 3NaCl + Fe(OH)_3$<br/><br/>The reaction is an example of________.
Question 302 :
What is the difference between displacement over double displacement reactions ?
Question 303 :
Which type of chemical decomposition reaction is the following reaction?<br>$Cu(OH)_2\xrightarrow {heat} CuO+H_2O$
Question 305 :
The conversion of ${Cr}_{2}{O}_{7}^{2-}$ to ${Cr}^{3+}$ during a chemical reaction is an example of:
Question 306 :
<p>Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of :</p>
Question 308 :
Which of the following symbol is used to show solid precipitate?
Question 310 :
The reaction of hydrochloric acid with zinc oxide to form zinc chloride and water is neutralization reaction. State whether the statement is true or false
Question 311 :
Other signs of a chemical reaction might be an increase in temperature if heat is released or a change in _____.
Question 313 :
When chlorine reacts with sodium bromide the solution turns brown because ________.
Question 314 :
Which of the following reaction requires oxygen to be a reactant ?
Question 315 :
Aqueous solutions of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate.<br/><br/>The balanced molecular equation contains which one of the following terms?
Question 322 :
Balanced chemical equations imply which of the following?<br>$I$. Numbers of molecules are conserved in chemical change<br>$II$. Numbers of atoms are conserved in chemical change.<br>$III$. Mass is conserved in chemical change.
Question 323 :
A chemical reaction in which heat is evolved is called a/an:<br/>
Question 327 :
Reaction of iron nails with copper sulphate solution is an example of ____________.
Question 329 :
State true or false.<br/><br/>In redox reactions, oxidation and reduction take place simultaneously.<br/>
Question 332 :
In which of the following equations, the reduction of metal oxides can be seen  :<br/>
Question 337 :
$CaC{ O }_{ 3 }\xrightarrow { \quad \quad \Delta \quad \quad  } CaO+C{ O }_{ 2 }\uparrow $ is a ________ reaction.
Question 338 :
Select the correct alternative.<br/>The reaction between acid and base to form salt and water is an example of :
Question 340 :
Magnesium reacts with acids producing hydrogen and corresponding magnesium salts. In such reactions Mg undergoes.
Question 342 :
Four hypothetical metallic elements $A, B, C$ and $D$ forms soluble nitrates having formulae $ANO_3, B(NO_3)_2, CNO_3$ and $D(NO_3)_3$.<br/>Strips of each of the four metals were immersed in $0.1\ M$ aqueous solution of the other metal nitrates, and the following observations recorded.<br/>1. Metal $B$ underwent reaction in all solutions.<br/>2. Metal $A$ only reacted with $CNO_3$.<br/>In order of increasing strength as reducing agents, the metals are :
Question 344 :
In the context of redox reactions, the gain of hydrogen from a substance is known as:
Question 347 :
Select the correct alternative.Which of the following is a decomposition reaction?
Question 348 :
When nitrogen reacts with hydrogen under high temperature and pressure to form ammonia gas. This is an example of:
Question 352 :
What would you observe when a mixture of iron filings and sulphur powder is heated strongly?<br/>
Question 354 :
<p>Ferric oxide reacts with aluminium to produce aluminium oxide and iron. The balanced chemical equation for the given reaction is :</p><p>$Fe_2O_3$ + $2Al$ $\rightarrow $ $Al_2O_3$ + $2Fe$ </p><p>Which of the following substances is oxidized in the given reaction?</p>
Question 355 :
Given $4NH_3(g)+5O_2(g)\rightarrow 4NO(g)+6H_2O(g)$<br>The above reaction is an example of:<br>(i)displacement reaction<br>(ii) combination reaction<br>(iii) redox reaction<br>(iv)neutralisation reaction
Question 357 :
Assertion: Silver metal does not show any change when it is placed in HCl solution.
Reason: Silver metal does not react with dilute HCl.
Question 358 :
Breaking of lead bromide into lead and bromine is an example of _____.
Question 359 :
The standard reduction potentials at 298 K for the following half reactions are given against each<br/>$Zn^{2+}(aq)+2e\rightleftharpoons Zn(s)\ -0.762$<br/>$Cr^{3+}(aq)+2e\rightleftharpoons Cr(s)\ -0.740$<br/>$2H^+(aq)+2e\rightleftharpoons H_2(g)\ 0.000$<br/>$Fe^{3+}(aq)+2e\rightleftharpoons Fe^{2+}(aq)\ 0.770$<br/>Which is the strongest reducing agent?
Question 360 :
In an oxidation process for a cell,<br>$M_1 \rightarrow{ M }_{ 1 }^{ n+ } + ne^- $<br>the other metal $(M_2)$ being univalent showing reduction takes up _____ electrons to complete redox reaction.
Question 361 :
The decomposition of mercuric oxide ($Hg)$) on heating, resulting in the formation of mercury and oxygen, is represented by the following chemical equation-<br/>$2HgO+180kJ\longrightarrow 2Hg+{O}_{2}$<br/>Which one of the following represents the heat of formation of mercuric oxide?
Question 362 :
Formation of nitric oxide from nitrogen and oxygen is a ______ reaction.
Question 363 :
Identify the product(s) which will be formed by using the information provided about the reactants.<br/>$NaCl(aq)+Ag{NO}_{3}(aq)\rightarrow$
Question 364 :
The standard reduction potentials at $25^{\circ}C$ for the following half reactions are given against each;<br>$Zn^{2+}(aq.) + 2e^{-}\rightleftharpoons Zn(s); -0.762$<br>$Cr^{3+}(aq.) + 3e^{-}\rightleftharpoons Cr(s); -0.740$<br>$2H^{+} + 2e^{-}\rightleftharpoons H_{2}(g); 0.00$<br>$Fe^{3+} + e^{-}\rightleftharpoons Fe^{2+}; 0.77$<br>Which is the strongest reducing agent?
Question 365 :
$\displaystyle CuO+{ H }_{ 2 }\longrightarrow { H }_{ 2 }O+Cu$, reaction is an example of :<br/>
Question 367 :
In a change hydrogen peroxide gives rise' to hydrogen oxide and oxygen. This is an example of:
Question 368 :
Exposure of silver bromide to sunlight for a long duration turns grey due to:
Question 370 :
Identify the product(s) which will be formed by using the information provided about the reactants :<br/>${C}_{4}{H}_{10}(g)+{O}_{2}(g) \rightarrow$
Question 371 :
On heating blue coloured powder of copper(II) nitrate in a boiling tube copper oxide (black), oxygen gas and a brown gas X is formed. Which type of reaction is this?<br/>
Question 372 :
Name the type of following chemical reaction.$KNO_3 + H_2SO_4 \rightarrow HNO_3 + KHSO_4$
Question 373 :
Which of the following are combination reactions?<br>(i)$2 \mathrm{KClO}_{3} \frac{\mathrm{Heat}}{\longrightarrow} 2 \mathrm{KCl}+3 \mathrm{O}_{2}$<br>(ii)$M g O+H_{2} o \rightarrow M g(O H)_{2}$<br>(iii)$4 A 1+3 O_{2} \rightarrow 2 A 1_{2} O_{3}$<br>(iv)$Z n+F e S O_{4} \rightarrow Z n S O_{4}+F e$
Question 374 :
The phenomenon of formation of simple sugar from starch in our body is the example of which reaction? 
Question 375 :
The reaction between carbon and oxygen can be represented as $C_{(s)}+O_{2(g)}\rightarrow CO_{2(g)}+heat$.<br/><br/>In which of the following type(s), the above reaction can be classified?<br/>I. Combustion reaction<br/>II. Displacement reaction<br/>III. Endothermic reaction<br/>IV. Combination reaction.<br/>
Question 376 :
When calcium nitrate thermally decomposes oxygen is one of the products. Which volume of oxygen is produced under room conditions when $0.50$ mol of calcium nitrate thermally decomposes?
Question 377 :
Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride. This is a :<br/>
Question 379 :
In which of the following compounds, an element exhibits two different oxidation states ?
Question 380 :
A metal ($A$) on heating in nitrogen gas gives compound $B$. $B$ on treatment with ${H}_{2}O$ gives a colourless gas which when passed through $Cu{SO}_{4}$ solution gives a dark blue-violet coloured solution. $A$ and $B$ respectively, are :
Question 381 :
A quantity of 5.68 g of pure $ P_{4}O_{10} $ is dissolved completely in sufficient water and the solution is diluted to 250 ml. Which of the following statement (s) is/are correct?
Question 382 :
Consider the following statements.<br/><br/>1. If all the reactants are not taken in their stoichiometric ratio, then at least one reactant will be left behind.<br/><br/>2. 2 moles of $H_2(g)$ and 3 moles of $O_2 (g)$ produce 2 moles of water.<br/><br/>3. If equal weights of carbon and oxygen are taken to produce $CO_2$, then $O_2$ is limiting reagent.<br/><br/>The above statements 1, 2, 3 respectively are (T = True, F = False):
Question 383 :
The reaction, when balanced, $m, n, p$ and $q$ are respectively:<br/>$K_2Cr_2O_7+ \ m FeSO_4+n H_2SO_4\rightarrow Cr_2(SO_4)_3+pFe_2(SO_4)_3+K_2SO_4+qH_2O$ 
Question 385 :
Which of the following chemical reaction is balanced as well as is combustion reaction?
Question 386 :
We know that balancing of a chemical equation is entirely based on the law of conservation of mass. However the concept of Principle of Atom Conservation (POAC) can also be related to law of conservation of a mass in a chemical reaction. So, POAC can also acts as a technique for balancing a chemical equation. For example, for a reaction $ABC_3 \rightarrow AB +C_2$ On applying POAC for A, B and C and relating the 3 equation we get : $\dfrac {n_{ABC_3}}{2}=\dfrac {n_{AB}}{2}=\dfrac {n_{C_2}}{3}$ (N= number of moles of X) Thus the coefficients of $ABC_3$, AB and $C_2$ in the balanced chemical equation will be 2,2 & 3 respectively and the balanced chemical equation can be represented as :$2ABC_3 \rightarrow 2AB +3C_2$Now answer the following question.<br/> <br/>If the weight ratio of C and $O_2$ present is 1:2 and both of the reactants completely cousumes and form CO and $CO_2$ and we will obtain a gaseous mixture of CO and $CO_2$ . What would be the weight ratio of CO and $CO_2$  in mixture?
Question 387 :
A quantity of $12\ g$ of magnesium is burnt completely in air $(O_{2}=20\%$ and $N_{2}=80\%$ by volume). Which of the following is/are correct statement(s) regarding this combustion?
Question 388 :
If the value of $n$ is $2$, then the atomic weight of metal $M$, is
Question 389 :
A quantity of $6\ g\ NaOH$ and $4.4\ g\ CO_{2}$ is allowed to react to form $Na_{2}CO_{3}$ or $NaHCO_{3}$ or both. Which of the following is correct statement regarding the reaction?
Question 392 :
An amount of 0.01 mole of $ SO_{2}Cl_{2} $ is hydrolysed completely in sufficientwater (no gas is allowed to escape out ) and the solution is diluted to 200 ml. Which of the following statement is/are correct? (Ag = 108 )
Question 393 :
What mass of ammonium nitrate should be heated to produce 8.96 litres of steam? [Note : Relative molecular mass of $NH_4NO_3$ is 80]
Question 394 :
What amount of $BaSO_4$ can be obtained on mixing 0.5 mole $BaCl_2$ with 1 mole of $H_2SO_4$ ?
Question 395 :
How much $CaO$ is to be added to the furnace charge for each $1280\ kg$ of pure $CaC_{2}$ produced ?
Question 397 :
A mixture of propane and benzene is burst completely in excess of oxygen at $110^{o}C$. It result the production of equal volumes of $CO_{2}(g)$ and steam (measured under identical pressure and temperature). Which of the following is correct regarding the original mixture?
Question 398 :
What is not a mole ratio of $ \displaystyle 6CO_2 + 6H_2O \rightarrow C_6H_{12}O_6 + 6O_2$? <br/>
Question 401 :
For the cell $Zn(s) |Zn^{2+} (aq) || M^{x+} (aq) | M(s)$, different half cells and their standard electrode potentials are given below:<br/><table class="wysiwyg-table"><tbody><tr><td>$M^{x+} (aq/M(s))$</td><td>$Au^{3+} (aq)/Au(s)$</td><td>$Ag^+ \, /Ag(s)$</td><td>$Fe^{3+}(aq)/Fe^{2+} (aq)$</td><td>$Fe^{2+}(aq)/Fe(s)$</td></tr><tr><td>$E^o_{M^{x+}/M^{/(V)}}$<br/></td><td>$1.40$</td><td>$0.80$</td><td>$0.77$</td><td>$-0.44$</td></tr></tbody></table><br/>If $E^o_{Zn^{2+}/Zn} = -0.76 V$, which cathode will give a maximum value of $E^o_{cell}$ per electron transferred ?
Question 403 :
For the reaction of sodium with water, the balanced equation using the smallest whole numbers has which of the following coefficients?<br/>I. $1$<br/>II. $2$<br/>III. $3$<br/>Select the correct option.<br/>
Question 404 :
What is the minimum mass of ${ CaCO }_{ 3 }(s)$, below which it decomposes completely, required to established equilibrium in a $6.50\ litre$ container for the reaction:<br/>${ CaCO }_{ 3 }(s)\rightleftharpoons CaO(s)+{ CO }_{ 2 }(g);\space { K }_{ c }=0.005\ mole/litre$<br/>
Question 405 :
Minimum amount of $Ag_2CO_3$ (s) required to produce sufficient amount of oxygen for the complete  combustion of $C_2H_2$ which produces 11.2 L of $CO_2$ at STP after combustion is_________.<br/>$Ag_2CO_3 (s) \, \rightarrow\, 2Ag (s)\, +\, CO_2 (g)\, +\, \dfrac12 O_2 (g)$<br/>$C_2H_2\, +\, \dfrac52 O_2\, \rightarrow\, 2CO_2\, +\, H_2O$<br/>
Question 407 :
How many grams of ammonium nitrate will be formed in the reaction?
Question 409 :
The equation which is balanced and represents the correct product is: