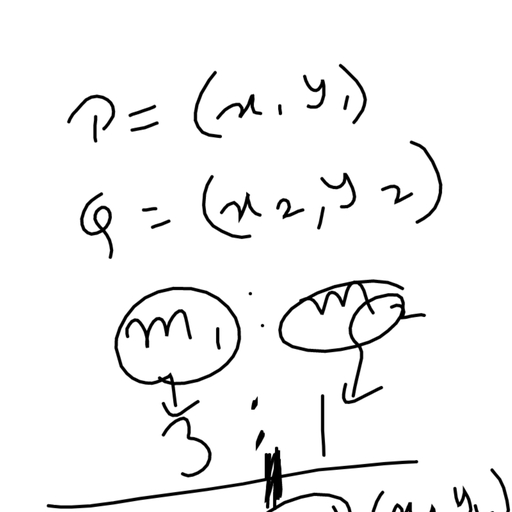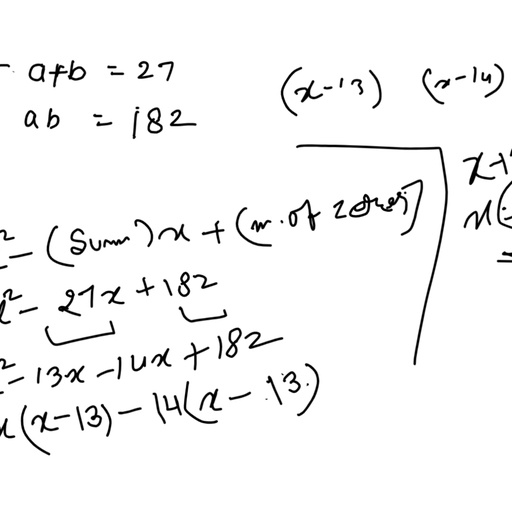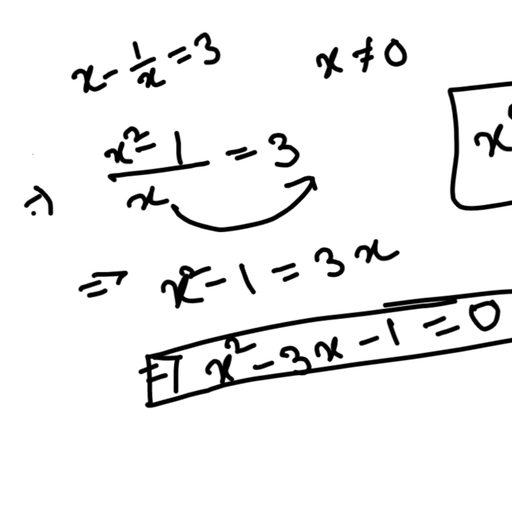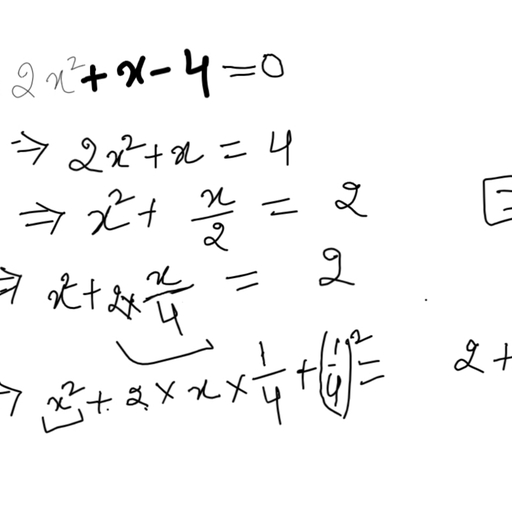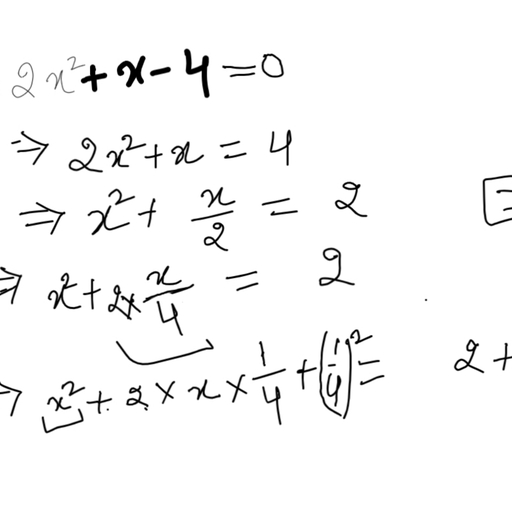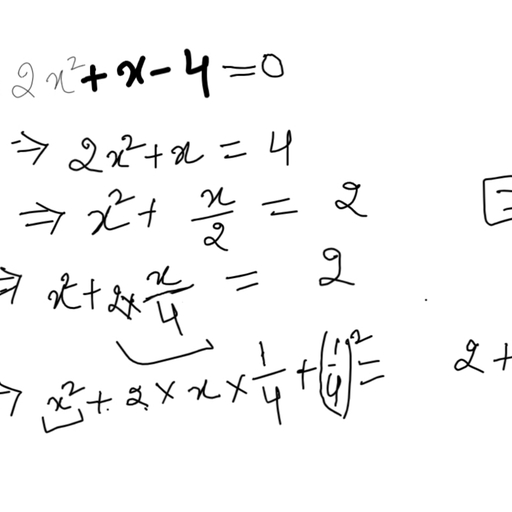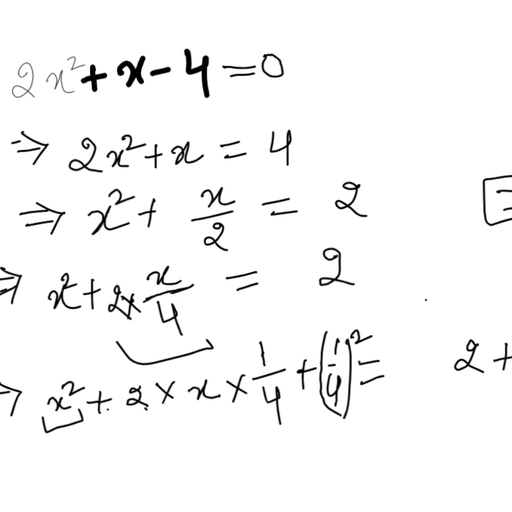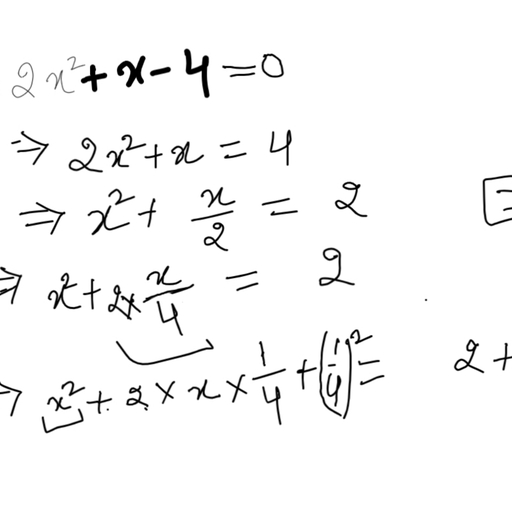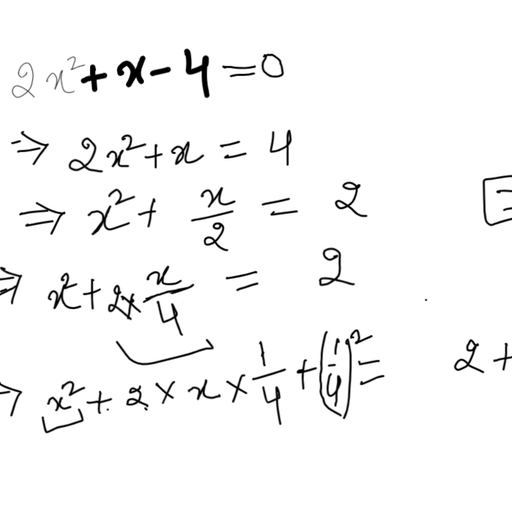Question 2 :
The chloride salt of a certain weak monoacidic organic base is hydrolysed to an extent of $3$% in its $0.1M$ solution at ${25}^{o}C$. Given that the ionic product of water is ${10}^{-14}$ at this temperature, what is the dissociation constant of the base?
Question 6 :
State whether the given statement is true or false:<br/>Bleaching powder contains about 50 % available chlorine.<br/>
Question 7 :
$NaHCO_{3}$ can be used on its own to make cakes or bread. Baking powder is more commonly used, and contains $NaHCO_{3}$, $Ca(H_{2}PO_{4})_{2}$ and starch. An improved combination baking powder contains about 40% starch, 30% $NaHCO_{3}$ 20% $NaAl(SO_{4})_{2}$ and 10% $Ca(H_{2}PO_{4})_{2}.$ Answer the following questions based on above study.<br/>Rise in bread and cakes is due to______ when it decomposes between $50^{o}C$ and $100^{o}C$. 
Question 8 :
The aqueous solution of which one of the following is basic?
Question 9 :
Galvanisation is a method of protecting iron from rusting, by coating with a thin layer of:<br/>
Question 10 :
In case of formation of ionic compounds, the electronegativity difference between them is less than 1.7.<br/>
Question 13 :
Amongst $LiCl$, $ BeCl_2,\ MgCl_2 $ and $RbCl$ the compounds with greatest and least ionic character, respectively are:
Question 14 :
Solubility of $NaCl$, $Na_2SO_4$ and $Na_3PO_4$ in water in increasing order is :
Question 15 :
Balanced chemical equations imply which of the following?<br>$I$. Numbers of molecules are conserved in chemical change<br>$II$. Numbers of atoms are conserved in chemical change.<br>$III$. Mass is conserved in chemical change.
Question 16 :
When chlorine reacts with sodium bromide the solution turns brown because ________.
Question 17 :
Which of the following reaction requires oxygen to be a reactant ?
Question 18 :
In the reaction, $4Fe+3O_2\rightleftharpoons 4Fe^{3+}+6O^{2-}$ Which of the following statements is incorrect?
Question 20 :
Which of the following reaction are disproportion reaction?<br/>a) $2Cu^+\rightarrow Cu^{2+}+Cu^o$<br/>b) $3MnO^{2-}_4+4H^+\rightarrow 2MnO_4^-+MnO_2+2H_2O$<br/>c) $2KMnO_4\xrightarrow []{\Delta}K_2MnO_4+MnO_2+O_2$<br/>d) $2MnO_4^-+3Mn^{2+}+2H_2O\rightarrow 5MnO_2+4H^{+}$
Question 21 :
In which of the following equations, the reduction of metal oxides can be seen  :<br/>
Question 23 :
$CaC{ O }_{ 3 }\xrightarrow { \quad \quad \Delta \quad \quad  } CaO+C{ O }_{ 2 }\uparrow $ is a ________ reaction.
Question 24 :
When magnesium burns in air, compound of magnesium formed are magnesium oxide and :
Question 25 :
A quantity of 5.68 g of pure $ P_{4}O_{10} $ is dissolved completely in sufficient water and the solution is diluted to 250 ml. Which of the following statement (s) is/are correct?