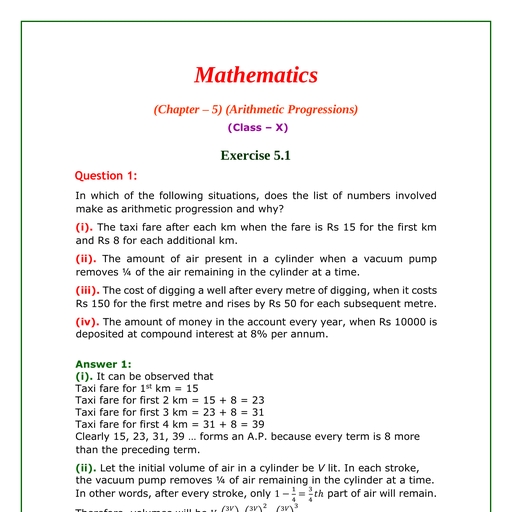
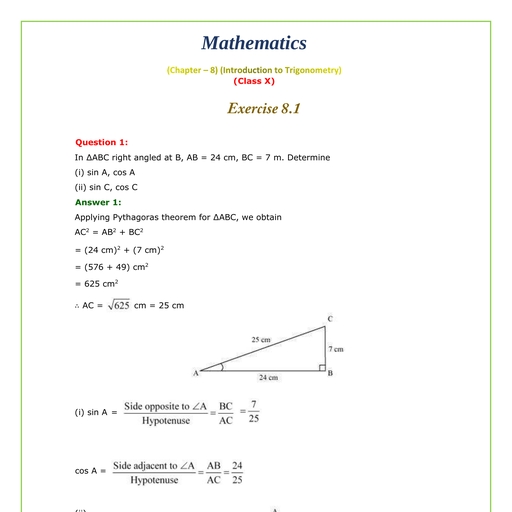
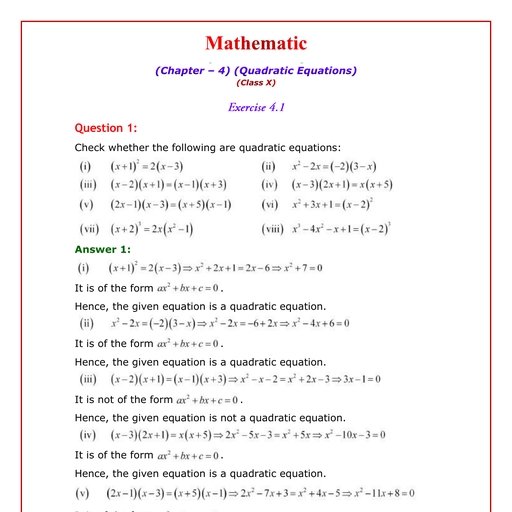
Question 1 :
The conduction of electricity through a solution involves the movement of_________ particles.
Question 3 :
How can you tell that a metal is reacting with water?
Question 8 :
<div><span>Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non-sonorous, non-malleable, and are colored.</span></div>Non-metal which is required for combustion is ______________.<br/>
Question 9 :
Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results were tabulated as below:<div><br/><table class="wysiwyg-table"><tbody><tr><td>Metal</td><td>Iron (II) Sulphate</td><td>Copper (II) sulphate</td><td>Zinc sulphate</td><td>Silver nitrate</td></tr><tr><td>A</td><td>No reaction</td><td>Displacement</td><td></td><td></td></tr><tr><td>B</td><td>Displacement</td><td></td><td>No reaction</td><td></td></tr><tr><td>C</td><td>No reaction</td><td>No reaction</td><td>No reaction</td><td>Displacement</td></tr><tr><td>D</td><td>No reaction</td><td>No reaction</td><td>No reaction</td><td>No reaction</td></tr></tbody></table><br/></div><div>Which is the most reactive metal?</div>
Question 14 :
During the formation of ionic bond ________transfer takes place from one atom to the others.
Question 16 :
Which of the following metals exist in their native state in nature?<br/>(i) Ca<br/>(ii) Au<br/>(iii) Zn<br/>(iv) Ag<br/>
Question 17 :
<span>A metal which reacts with cold water vigorously producing hydrogen is:<br/></span>
Question 18 :
<span>During extraction of metals, electrolytic refining is used to obtain pure metals. </span>Material which will be used as anode, for refining of silver metal by this process, is:<br/>
Question 19 :
Arrange the following metals in the decreasing order of their chemical reactivity placing the most active first. The metals are <span>Cu, Mg, Fe, Na, Ca, Zn.</span>
Question 20 :
Name two product of the given reaction.<div><span>$Zn   + \underset{(Steam)}{H_2O}    \rightarrow       $</span><br/></div>
Question 21 :
The metals sodium, calcium, and magnesium at the top of the reactivity series can be obtained by:
Question 22 :
When sodium reacts with cold water, then the product formed will be:
Question 24 :
Metals in the middle of the activity series are generally present as _______ in nature.
Question 25 :
<div>$Fe_{x}O_{3}(s) + 2Al(s) \rightarrow  2Fe(l) + Al_{2}O_{y}(s) + Heat$<br/>What are the values of $x$ and $y$?</div>
Question 26 :
Minerals that have a relatively high concentration of metal and can be extracted profitably from them are called:
Question 30 :
At room temperature an example of a liquid metal is ______and that of a liquid non-metal is______ .
Question 34 :
Which of the following is the only liquid metal which is a poor conductor of heat, but a fair conductor of electricity?
Question 36 :
<div><span>Metals are usually hard and difficult to cut.</span><br/><span>On the other hand, non-metals are usually brittle. When hit with a hammer, they break into small pieces.</span><br/></div><div><br/></div>Identify the element that is relatively more brittle from the options below:
Question 41 :
Which metal is used to connect solar cell to solar panels?
Question 42 :
<b><span>The acid solution is taken in two test tubes. Zinc is introduced in the first test tube and magnesium in the second. Gas is evolved in the second test tube which burns with a pop sound while no gas is evolved from the first test tube. Identify the acid solution taken in the two test tubes.</span></b><br/>
Question 43 :
Silver articles become black on prolonged exposure to air. This is due to the formation of
Question 45 :
Fill in the blanks by selecting the option with the correct words.<br/>The process of removing impurities from the impure metal to obtain pure metal is known as _____of metal. The most widely used method for refining is _________. In this method, the impure metal is taken as ________ and the pure metal is taken as _____. 
Question 46 :
The gas given off when a metal reacts with an acid is
Question 47 :
Mark the correct statements:<br/><br/><div>(i) mercury can be refined by the process of distillation.<br/><br/></div><div>(ii) In poling, the molten impure metal is stirred with green poles of wood.<br/><br/></div><div>(iii) In electrolyte refining of metals, impure metal is made as cathode and a thin strip of pure metal is made as anode.</div>
Question 50 :
A metal gives lilac colour in bunsen flame and the solution of its oxide turns red litmus paper blue. The metal is?