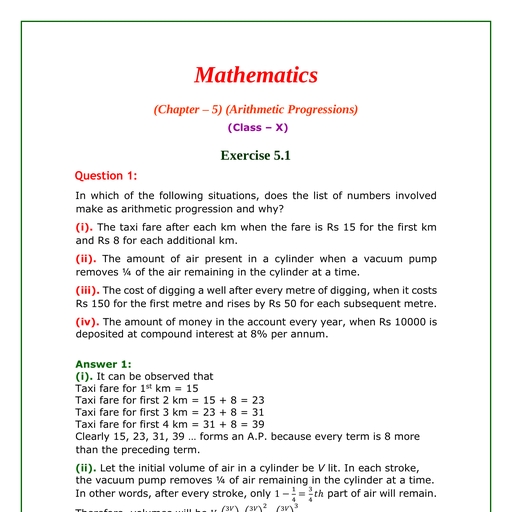
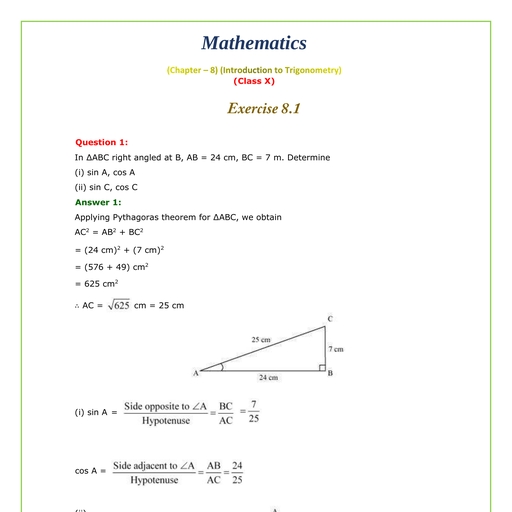
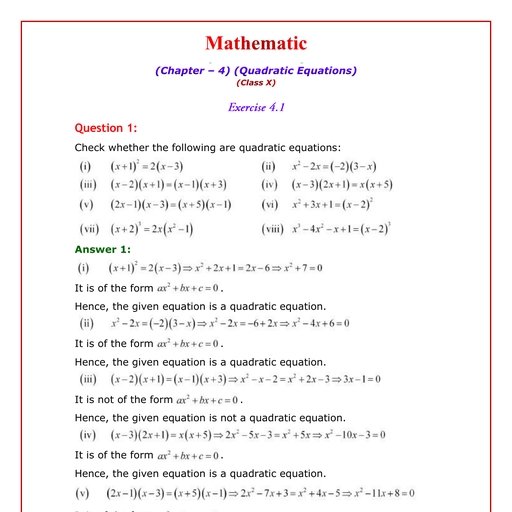
Question 1 :
In which group, all the elements do not have the same number of valence electrons?<br/>
Question 2 :
The name given by Newlands to the arrangement created by him was:
Question 7 :
How did Mendeleev predict the existence of some elements yet to be discovered elements in his periodic table?<br/>
Question 9 :
Which element has a dicey position in the modern periodic table?
Question 10 :
In the first two groups of periodic table, the group number represents the :<br/>
Question 12 :
Which of the following statements about Mendeleev's Periodic Table is incorrect?
Question 13 :
Which of the following triads does not follow Dobereiner's law of triads?
Question 14 :
An element which is an inert gas with atomic number 2 is :<br/>
Question 15 :
Mendeleev used a Sanskrit numeral before the names of the predicted elements. What was the term used for it?
Question 16 :
Which periods were not divided into two series in the Mendeleev's periodic table?
Question 17 :
Which of the following element(s) was/were not known when Mendeleev gave his classification?
Question 18 :
The eighth group of Mendeleev's periodic table is divided into how many subgroups?
Question 20 :
As we move along a period, the valency of elements :
Question 22 :
What happens to the number of valence electrons in atoms of elements as we go down a group in the periodic table?
Question 24 :
The number of elements known in Mendeleev's presented periodic table is:<br/>
Question 29 :
The placement of elements in the Newlands' table was compared to:
Question 31 :
The early attempt to classify elements as metals and non-metals was made by :
Question 33 :
Cl, Br, I  follows Dobereiner's triads, the atomic masses of Cl and I are 35.5 and 127 respectively. The atomic mass of Br is : <br/>
Question 35 :
In Newlands classification, elements are arranged in an increasing order of their:
Question 36 :
An element with electronic configuration $2,\,8,\,2$ belongs to which period and group?
Question 38 :
The electronic configuration of calcium is $2,\,8,\,8,\,2$. Find the valency of the element.
Question 40 :
The group in which all the elements do not have same number electrons in their valence shell is :
Question 41 :
The groups from $1^{st}$ to $7^{th}$ of Mendeleev's periodic table were divided into ______ subgroups.
Question 44 :
John Newlands arranged the known elements in a/an  ______ order of their atomic _______ .<br/>
Question 45 :
For which of the pair Newland octave rule is not applicable?
Question 48 :
Which one of the following is an example of Dobereiner's triad?
Question 49 :
Which of the following elements has the maximum number of outermost electrons?
Question 51 :
Atomic number of an element is 16. What is the nature of the element?
Question 52 :
On moving from top to bottom in a group in the periodic table, valency:
Question 53 :
The Newland's law of octaves for the classification of elements was found to be applicable only up to the element ______________________.
Question 56 :
The group number of element with atomic number 7 in the periodic table is:<br/>
Question 58 :
According to the "Law of Octaves" the properties of lithium resembles with the:<br/>
Question 59 :
The most important active step in the development of periodic table was taken by
Question 60 :
Dobereiner was the first person to illustrate the relationship between the ____________ of elements and their properties.
Question 63 :
If three elements $X, Y,$ and $Z$ form a Dobereiner's triad and atomic weights of $X$ and $Z$ are $9$ and $40$ respectively, then the atomic weight of the element $Y$ is approximate:
Question 64 :
In a triad of $A, B, C$ elements, if the atomic masses of $A$ and $C$ respectively are $100$ and $200$, then the atomic mass of $B$ is:
Question 65 :
Which of the following is a defect in the Mendeleev's periodic table?
Question 66 :
Which of the following statements are correct about Mendeleev's periodic table?<br/>i) It is based on increasing order of atomic numbers.<br/>ii) Mendeleev corrected the atomic weight of some elements like Be, In etc.<br/>iii) $(Ar , H_{2} ), (Co, Cl_{2}), (Te, F_{2})$ are three inverted pairs.<br/>iv) It is based on increasing order of atomic weights.
Question 67 :
Which of the following is not a merit of Mendeleev's periodic table?
Question 68 :
If A, B and C are the three elements of Dobereiner's triad and atomic weights of A and B are 7 and 15 respectively, then the atomic weight of C is :
Question 69 :
Which of the following can be classified as Dobereiner's triads?<div>(a) P, As, Sb <div>(b) Cu, Ag, Au</div><div><span>(c) S, Se, Te<br/></span></div></div>
Question 70 :
Which of the following is arranged in order of increasing metallic character?
Question 71 :
The elements $A$ and $B$ obey Newland's law of octaves. How many elements are there between $A$ and $B$?
Question 73 :
Whose name is not associated with the development of periodic table?
Question 74 :
Name the following with reference to the elements of the modern periodic table.<br/><br/>The alkali metal in period 2 
Question 76 :
The valency of elements in the periodic table with respect to hydrogen:
Question 77 :
Which is not anomalous pair of elements in the Mendeleev's periodic table?
Question 78 :
Which element resembles cobalt and nickel but was placed in Newlands' table far away from those elements?
Question 80 :
According to the Newlands' law of octaves, the properties of fluorine are similar to those of  ________ .<br/>
Question 81 :
Which group of elements could be placed in Mendeleev's Periodic Table without disturbing the original order?<br/>
Question 82 :
An element which is an essential constituent of all organic compounds belongs to :<br>
Question 83 :
Doberiener's system of classification into triads was not found to be useful as he could identify only:
Question 86 :
Arrange the following elements in the order of their increasing non-metallic character:<div><br/>Li, Be, C, O, F<br/></div>
Question 87 :
Which of the following properties generally shows a decreasing trend along a period?
Question 88 :
Which of the following accounts best for the fact that fluorine atom is smaller than that of oxygen?
Question 89 :
<SPAN>Based on general trends in the periodic table, predict which element in each of the following pairs has the most metallic character.<BR>(i) Sn or Pb (ii) Ag or Sr (iii) Al or B (iv) Br or As</SPAN>