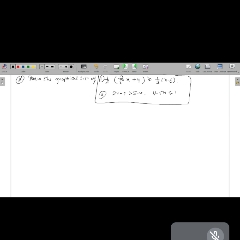
Question 1 :
Identify the unsaturated compounds from the following :<br/><span>$(i)$ Propane $(ii)$ Propene $(iii)$ Propyne $(iv)$ 2-Chloropropane</span>
Question 2 :
Given compounds: propyne, acetylene, butane, butanol, propene, ethane, and ethene. How many of these compounds are unsaturated?
Question 3 :
Wet ether is not used as a solvent in Wurtz reaction because of the water present in it_____________.<br/>
Question 4 :
According to Huckel's rule, a compound is said to be aromatic, if it contains:<br/>
Question 5 :
In which of the following pair both angle strain and torsional strain is present?
Question 6 :
The necessary condition for the aromatic ring is that it should have .................$\displaystyle \pi -$electrons.<br/>
Question 7 :
The alternative path for radical chain reactions of $Br_{2}$ with ethane may be represented as follows:<br/>1) $ CH_{3}-CH_{3}+Br_{2}\rightarrow CH_{3}-CH_{2} -Br+HBr$<br/>2) $CH_{3}-CH_{3}+Br_{2} \rightarrow 2CH_{3}Br$<br/>
Question 8 :
The reaction ${ C }_{ 12 }{ H }_{ 26 }\rightarrow { C }_{ 6 }{ H }_{ 12 }+{ C }_{ 6 }{ H }_{ 14 }$ represents :
Question 9 :
Torsional strain in eclipsed conformations of a molecule is due to:<br/>
Question 10 :
The discovery that chlorofluorocarbons initiate a radical -chain reaction that catalytically destroys ozone won the 1995Nobel prize in chemistry Given the initiation step below which of the following reactions is the most likely to serve as one of the propagation steps?<br>$CCl_{2}F_{2}+hv\rightarrow ^{*} CClF_{2}+^{*}Cl$<br>
Question 11 :
$C_6H_6\xrightarrow[HNO_3]{H_2SO_4} A$.In the given raection which of the following is the product A:<br/>
Question 12 :
Which of the following compounds will not undergo Friedel-Craft reaction easily?
Question 16 :
The number of total product produced upon monochloriation of (+) 2-chlorobutane is ___________.
Question 17 :
Identify the correct order of reactivity in electrophilic substitution reaction of the following compounds <br>1. Benzene, 2. Toluene, 3. Chlorobenzene, 4. Nitrobenzene<br>
Question 18 :
In the reaction $CH_3COONa\, +\, NaOH\, \xrightarrow[Heat]{CaO}\, X\, +\, Na_2CO_3$, product 'X' would be :
Question 21 :
Which of the following statements is not correct regarding aromatic compounds?
Question 22 :
What effect does branching of an alkane chain has on its melting point ?
Question 24 :
A five carbon chain with a double bond would be named :
Question 26 :
Which of the following has the maximum $C - H$ bond length?<br/>
Question 27 :
Which of the following is soluble in methanol:<br/>I : Hexane <div><span>II : Cyclohexane </span><div><div>III : $2-methyl pentane$ </div><div><span>IV : Chloroethane</span></div></div></div>
Question 28 :
Statement-I: Iodination of alkanes is carried out in the presence of iodic acid.<br/>Because<br/>Statement-II: Iodic acid removes $I_2$ gas from the reaction mixture.<br/>
Question 30 :
Which of the following is the most reactive towards ring nitration?
Question 32 :
Which one of the following compounds gives methane on treatment with water?
Question 34 :
What is correct order of rate of nitration of the following compounds?<br>$\underset{A}{C_6H_5CH_3}$ $\underset{B}{C_6H_6}$ $\underset{C}{C_6D_6}$ $\underset{D}{C_6T_6}$ $\underset{E}{C_6H_5Br_3}$ $\underset{F}{C_6H_5NR_3}$ $\underset{G}{C_6H_5NMe_2}$.<br>
Question 35 :
A saturated hydrocarbon is shown by ${ C }_{ n }{ H }_{ 20 }$ . The value of $n$ in this compound is:<br/>
Question 36 :
Which of the following will produce a pair of diastereomers on free radical chlorination giving a dichloro derivative?<br>
Question 40 :
A hydrocarbon (A) was found to have vapour density 36. It forms only single mono chlorosubstitution product.Suggest (A).
Question 46 :
Arrange the following in the decreasing order of their melting points.<br>I. Decane<br>II. Nonane<br>III. Octane<br>IV. Heptane
Question 49 :
A given compound satisfies one or more of the following criteria. <br/>(A) No pi electrons are present.<br/>(B) Pi electrons are not delocalised.<br/>(C) The compound has non-planar structure.<br/>What can be said about this compound?<br/>