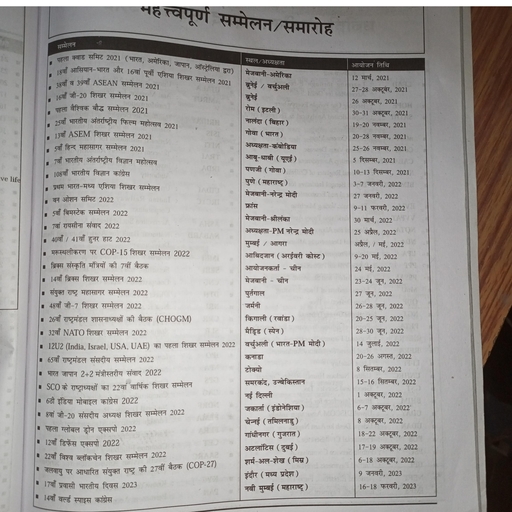
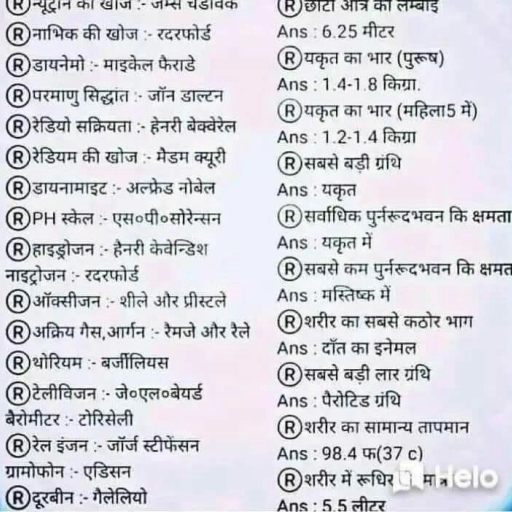
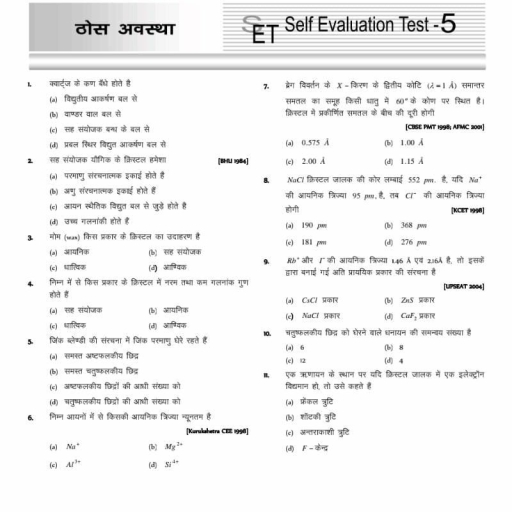
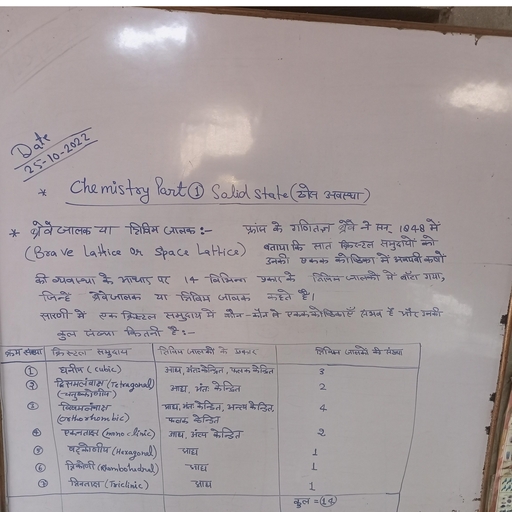
Question 1 :
The result of the operation 2.5 X 1.25 should be which of the following on the basis of significant figures?
Question 3 :
Assertion: The number of significant figures in 507000 is three.<br/>
Reason: In 507000, all the zeros are significant. 
Question 4 :
On dividing 0.46 by 15.374, the actual answer is 0.02992. The correctly reported answer will be:<br/>
Question 5 :
Assertion: Significant figures for $0.200$ is $3$ whereas for $200$ it is $1$
Reason: Zero at the end or right of a number are significant provided they are not on the right side of the decimal point.
Question 11 :
If repeated measurements give values close to one another, the number is:
Question 13 :
Two student performed the same experiment separately and each one of them recorded two readings of mass which are given below. Correct reading of mass is $3.0\ g$. One the basis of given data, mark the correct option out of the following statement.<br><table class="wysiwyg-table"><tbody><tr><td>Student</td><td>Reading</td></tr><tr><td>$A$</td><td>$3.01\quad 2.99$</td></tr><tr><td>$B$</td><td>$3.05\quad 2.95$</td></tr></tbody></table>
Question 16 :
The prefix used for denoting an order of magnitude of ${ 10 }^{ 6 }$ is:<br/>
Question 17 :
According to the Avogadro's Law, equal volumes of two different gases, under same conditions of temperature and pressure, contain equal number of:<br/>
Question 18 :
Which statement is linked with the idea that two identical containers filled with different gases will contain the same number of particles?
Question 19 :
Chemical reactions such as redox reactions involve transfer of ___________one species to other species.<br/>
Question 20 :
Which of the following statements is the most accurate with regard to the significance of Avogadro's number, $6.02 \times 10^{23}$ ?
Question 21 :
Two samples of lead oxide were separately reduced to metallic lead by heating in a current of hydrogen. The weight of lead from one oxide was half the weight of lead obtained from the other oxide. The data illustrates. <br>
Question 22 :
Who laid the foundation of chemical sciences by establishing two important laws of chemical combination ?
Question 23 :
______ is a branch of chemistry in which quantitative relationship between masses of reactants and products are established. 
Question 24 :
French chemist ____ laid the foundation to the scientific investigation of matter by describing that substances react by following certain laws.<br/>
Question 26 :
The number of molecules in 1 L of oxygen and 1 L of nitrogen at STP will be:
Question 29 :
Matter is neither created nor destroyed during any physical or chemical change. This statement is :
Question 30 :
The chemical equation are balanced to satisfy one of the following laws in chemical reactions. This law is known as:
Question 31 :
The energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of:<br/>
Question 32 :
A chemical equation is balanced in accordance with the law of
Question 33 :
Which of the following is true with respect to Law of Conservation of matter?
Question 34 :
When mass is enclosed in a system and none is allowed in or out, it's _____ will never change.
Question 35 :
A balanced chemical equation is in accordance with the law of conservation of masses___________.
Question 37 :
Which one of the following laws directly explains the law of conservation of mass?<br/>
Question 38 :
Which gas law relates the volume of a gas to the number of molecules of the gas?<br/>
Question 39 :
Find the total number of pennies in a tenth of a mole of pennies.
Question 40 :
A sample of ammonia has a mass of $51.1g$. How many molecules are in this sample?
Question 41 :
How many moles are present in $6.023\times 10^{22}$ molecules of $CO_2$?<br>
Question 42 :
What is the volume of $3.0\times {10}^{20}$ molecules of $HCl (g)$?
Question 43 :
The critical volume of a gas is 0.036 $lit. mol^{-1}$. The radius of the molecule will be (in cm):<br/>(Avogadro Number = $6 \times 10^{23}$)<br/>
Question 44 :
Law of definite proportion does not apply to nitrogen oxide because:
Question 45 :
If the ratio of the atoms by mass is altered then :
Question 47 :
In a compound the ratio of the atoms or element by mass remains always same irrespective of :<br/>
Question 48 :
Different samples of water were found to contain hydrogen and oxygen in the ratio of 1 : 8. This shows the law of:<br/>
Question 49 :
The ratio of mass of nitrogen and oxygen in nitric oxide is in :
Question 50 :
The % of Copper and Oxygen in samples of CuO obtained by different methods were found to be the same. This proves the law of:<br/>
Question 51 :
If water samples are taken from the sea, rivers, clouds, lakes or snow, they will be found to contain hydrogen and oxygen in the ration of $1 : 8$. This clearly demonstrates the law of ?
Question 52 :
Assertion: Scientific notation for the number 100 is expressed as $ 10^2$.
Reason: The number $10^2$ has two significant figures.
Question 53 :
In the reaction, $N_2 + 3H_2 \rightarrow 2NH_3$, the ratio of volumes of nitrogen, hydrogen and ammonia is 1 : 3: 2. These figures illustrate the law of: <br>
Question 54 :
What mass of sodium chloride would be decomposed by 9.8 g of sulphuric acid if 12 g of sodium bisulphate and 2.75 g of hydrogen chloride were produced in a reaction?
Question 55 :
Assertion: Pure water obtained from different sources such as river, well, spring, sea etc. always contain hydrogen and oxygen combined in the ratio $1 : 8$ by mass.
Reason: A chemical compound always contains elements combined together in same proportion by mass, it was discovered by French chemist, Joseph Proust (1799).
Question 56 :
How many molecules of water are present in a $0.25\ mole$ of $H_2O$?
Question 59 :
Assertion: Atoms can neither be created nor destroyed.
Reason: Under similar condition of temperature and pressure, equal volume of gases does not contain equal number of atoms.
Question 61 :
The ratio of the dimension of Planck's constant and that of the moment of inertia is the dimension of :
Question 62 :
The actual product of 4.327 and 2.8 is 12.1156. The correctly reported answer will be :<br/>
Question 63 :
How many atoms of hydrogen are present in $7.8\ g$ of $Al{(OH)}_{3}$?
Question 65 :
A vessel contains X number of molecules of hydrogen gas at a certain temperature and pressure. Under the same conditions of temperature and pressure, how many molecules of nitrogen gas would be present in the same vessel?
Question 67 :
Mark the rule which is not correctly stated about the determination of significant figures.
Question 68 :
<p>A sealed tube containing phosphorus was weighed and heated carefully. After completion of the reaction, the tube was re-weighed under similar conditions. The weight of the tube will:</p>
Question 69 :
<p>The law of conservation of mass will not be strictly valid unless mass and energy are considered together.</p>
Question 70 :
Assertion: Avogadro's law hold's good only under similar conditions of temperature and pressure.
Reason: Change in temperature or pressure brings about change in volume.
Question 71 :
Assertion: Matter can neither be created nor destroyed.
Reason: This is law of definite proportions.
Question 72 :
4.88 g of $KClO_3$ when heated produced 1.92 g of $O_2$ and 2.96 g of KCl. Which of the following statements regarding the experiment is correct?
Question 73 :
The mass of hydrogen at STP, that is present in a vessel which can hold $4$ grams of oxygen under similar conditions, is:<br/>
Question 74 :
Combustion of a piece of paper to form ash, water vapour and carbon dioxide follows law of conservation of mass.<br/>
Question 75 :
$AgNO_3+NaCl\rightarrow AgCl+NaNO_3$. In this reaction, the mass of $AgNO_3$ and $NaCl$ is equal the mass of AgCl and $NaNO_3$. Then this chemical reaction satisfies :
Question 76 :
$2.16$ grams of Cu, on reaction with $HNO_{3}$, followed by ignition of the nitrate, gave $2.7$ g of copper oxide. In another experiment $1.15$ g of copper oxide, upon reaction with hydrogen, gave $0.92$ g of copper. This data illustrate the law of:<br/>
Question 77 :
Which scientist discovered that the same amount of space was occupied by equal numbers of molecules of gases, irrespective of whether it was hydrogen or chlorine or fluorine?
Question 78 :
Assertion: Pure water obtained from different states of India always contains hydrogen and oxygen in the ratio of $1:8$ by mass.
Reason: Total mass of reactants and products during chemical change is always the same.
Question 79 :
Given the numbers $786$, $0.786$ and $0.0786$. The number significant figures for the numbers is :<br/>
Question 80 :
14 g of element X combine with 16 g of oxygen. On the basis of this information, which of the following is a correct statement?
Question 81 :
Which of the following is a suitable example for illustrating the law of conservation of mass? (Atomic mass of O = 16; H = 1)
Question 82 :
Which choice shows the answer to the following mathematical operation with the correct number of significant figures?<br/>$2.8\times 8.96 = ? $
Question 83 :
The law which states that in a chemical reaction, the total mass of the products is equal to the total mass of the reactants
Question 85 :
One gram molecule of any gas at $NTP$ occupies $22.4\ L$. This fact was derived from:
Question 86 :
A sample was weighted using two different balances. The result's were (i) $3.929g$ (ii) $4.0g$. How would the weight of the sample be reported?
Question 87 :
Which of the following is a suitable example for illustrating the law of conservation of mass? (Atomic mass of $O=16; H=1$)<br/>
Question 88 :
The haemoglobin contains $0.33$% of iron by weight. Its molecular weight is $67200$. What is the number of iron atoms in each molecule (atomic weight of iron$=56$)?<br>
Question 90 :
10.0 g of CaCO$_3$ on heating gave 4.4 g of CO$_2$ and 5.6 g of CaO. The observation is in agreement with the:
Question 91 :
Assertion: One mole of oxygen gas occupies $22.4\ l$ volume at STP.
Reason: Volume of a gas depends on temperature and pressure and also nature of gas.
Question 92 :
Which choice shows the answer to the following mathematical operation with the correct number of significant figures?<br>$14.5-3.46=$______
Question 93 :
According to Avogadro's hypothesis, equal volumes of gases under the same conditions of temperature and pressure will contain:
Question 94 :
Assertion: Specific gravity is dimensionless quantity.
Reason:  Specific gravity is relative density of a substance, measured with respect to density of water at $4^o C$. 
Question 96 :
The law which states that in a chemical reaction, the total mass of the product is equal to the total mass of the reactants.
Question 98 :
In an experiment, 2.4 g of iron oxide on reduction with hydrogen gave 1.68 g of iron. In another experiment, 2.9 g of iron oxide gave 2.09 g of iron on reduction. Which law is illustrated from the above data?
Question 100 :
Statement 1: The number of gram molecules of oxygen in $6.02 \times 10^{24}\ CO$ molecules is 5.0.<br/>Statement 2: The value of Avogadro number is $6.02 \times 10^{23}$
Question 101 :
The mass of oxygen with which 13.5 g of aluminium will completely react is:
Question 102 :
Zinc sulphate contains $22.65$% $Zn$ and $43.9$% ${H}_{2}O$. If the law of constant proportions is true, then the mass of zinc required to give $40g$ crystal will be:
Question 103 :
Which of the statements are true about the law of chemical combination?<br>
Question 105 :
When 100 ml of a $O_{2} - O_{3}$ mixture was passed through turpentine, there was the reduction of volume by 20 ml. If 100ml of such a mixture is heated, what will be the increase in volume?
Question 106 :
$1.375$ g of cupric oxide was reduced by heating in a current of hydrogen and the weight of copper obtained was $1.098$ g. In another experiment, $1.179$ g of copper was dissolved in nitric acid and the resulting solution was evaporated to dryness. The residue of copper nitrate when strongly heated was converted into $1.4476$ g of cupric oxide. The result of this process show :
Question 107 :
At NTP, 10 litre of hydrogen sulphide gas reacted with 10 litre of sulphur dioxide gas. The volume of gas, after the reaction is complete, would be:
Question 108 :
The mass of residue left after strongly heating 1.38 g of silver carbonate will be:
Question 109 :
$14$ g of element X combines with $16$ g of oxygen. On the basis of this information, which of the following is a correct statement?
Question 110 :
$10$ mL of hydrogen combines with $5$ mL of oxygen to yield water. When $200$ mL of hydrogen at STP is passed over heated $CuO$, the $CuO$ loses $0.144$ g of its weight. State the law illustrated by these chemical combinations.
Question 111 :
In a gaseous reaction of the type, $aA + bB \rightarrow  cC + dD$, which is wrong?<br/>
Question 112 :
Statement 1: At the same temperature and pressure, 1 L of hydrogen gas and  1 L of neon gas have the same mass.<br/>Statement 2: Equal volumes of ideal gases at the same temperature and pressure contain the same number of moles.
Question 113 :
Which of the following statements is correct about the reaction given below?<br>$\displaystyle 4Fe\left ( s \right )+3O_{2}\left ( g \right )\rightarrow 2Fe_{2}O_{3}\left ( g \right )$<br>
Question 114 :
A student performs a titration with different burettes and finds titre values of $25.2$ ml, $25.25$ ml and $25.0$ ml. The number of significant figures in the average titre value is :
Question 115 :
Which of the following is a suitable example for illustrating the law of conservation of mass? (Atomic mass of $O= 16,H= 1$ g/mole)<br/>
Question 116 :
The value of observed and calculated molecular weight of silver nitrate is 92.64 and 170 respectively the degree of dissociation of silver nitrate is:
Question 117 :
$CaCO_3 + 2HCl \rightarrow CaCl_2 + H_2O + CO_2$<br/>The volume of CO$_2$ gas formed when 2.5 g calcium carbonate is dissolved in excess hydrochloric acid at 0$^o$C and 1 atm pressure is:[1 mole of any gas at 0$^o$C and 1 atm pressure occupies 22.414 L volume].
Question 118 :
Calculate the volume of $CO_{2}$ evolved by the combustion of $50$ ml of a mixture containing $40$ per $C_{2}H_{4}$ and $60$ per $CH_{4}$ (by volume).
Question 119 :
If $224ml$ of triatomic gas has a mass of $1g$ at $273K$ and $1atm$ pressure, then the mass of one atom is-
Question 121 :
Assertion: STATEMENT 1: $SO_{2}$ obtained either by the oxidation of $H_{2}S$ or decomposition of sulphate salt contains sulphur and oxygen in the mass ratio $1 : 2.$
Reason: STATEMENT 2: $S_{(s)}+O_{2(g)}\rightarrow SO_{2(g).}$
Question 122 :
Percentage purity of a sample of gold is $85\%$. How many atoms of gold are present in its $1$ gram sample? (Atomic mass of gold $=197$u).
Question 123 :
20 ml of a mixture of $C_{2}H_{2}$ and CO was exploded with 30ml of oxygen. The gases after the reaction had a volume of 34ml. On treatement with $KOH$, 8ml of oxygen remained. Calculate the composition of the mixture.
Question 124 :
Hydrogen and oxygen combine to form $H_{2}O_{2}$ and $H_{2}O$ containing $5.93\%$ and $11.2\%$ the respectively. The data illustrates the:
Question 125 :
A measured mass of an unreactive metal was dropped into a small graduated cylinder half filled with water. The following measurement were made:<br/>Mass of metal $= 25.200\ g$<br/>Volume of water before addition of metal $=12.5\ mL$<br/>Volume of water after addition of metal $= 15.0\ mL$<br/>The density of the metal should be reported as:
Question 126 :
10 ml of CO is mixed with 25 ml air having  20 per cent $O_{2}$ by volume. What would be the final volume if none of CO and $\displaystyle\: O_{2}$ is left after the reaction?
Question 127 :
<p><b> 60 g</b> of a compound on analysis produced <b>24 g </b><b> </b><b>carbon</b>, <b>4 g </b><b> </b><b>hydrogen</b>, and <b> 32 g</b><b> </b><b>oxygen</b>. The empirical formula of the compound is</p>
Question 128 :
40 ml of mixture of $C_{2}H_{2}$ and CO is mixed with 100 ml of $O_{2}$ gas and the mixture is exploded. The residual gases occupied 104 ml and when these are passed through KOH solution, the volume becomes 48ml. All the volume are at same the temperature and pressure. Determine the composition of the original mixture.
Question 130 :
The number of $H_{3}O^{+}$ ions present in 10ml of water at $25^{\circ}C$ is:<br/>
Question 131 :
Assertion: Carbon monoxide is considered a compound but carbon and oxygen as elements.
Reason: A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number, which is the number of protons in its atomic nucleus. While the compound is composed of two or more separate elements.
Question 132 :
What volume of hydrogen at NTP will be liberated when 3.25 g of zinc completely dissolve in dilute HCl? <br/>[At. mass of Zn = 65]<br/>
Question 133 :
Which units of pressure are needed if you are going to use 0.0821 as your ideal gas constant?
Question 134 :
10 ml of a mixture of $CH_{4}, C_{2}H_{4}$ and $CO_{2}$ were exploded with excess of air. After explosion, there was contraction on cooling of 17 ml and after treatment with KOH, there was further reduction of 14 ml. What is the composition of the mixture?
Question 135 :
The vapours of organic compound was burnt in oxygen. Equal volume of both gaseous substance were taken at same pressure and temperature. After the reaction, the system was returned to the original condition and it turn out that its volume has not changed. The product of combustion contain 50% $CO_{2}$(g) and 50% $H_{2}O$(g) by volume and no other gas. Find the molecular weight of organic compound (in gram/mol) in question.
Question 136 :
In the relation, $p={ \dfrac { \alpha  }{ \beta  } { e }^{ -\dfrac { \alpha Z }{ k\theta  }  } }$ <br/>$P$ is pressure, $Z$ is distance, $k$ is Boltzmann's constant and $\theta$ is the temperature. The dimensional formula of $\alpha$ will be:
Question 137 :
A salt is formed due to the reaction between an oxy acid containing chlorine and a base containing a monovalent metal of atomic mass $x$. The number of oxygen atoms in one molecule of the acid is more than the corresponding 'ic' acid. Calculate the molecular mass of the salt.