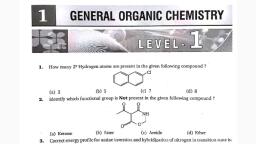
Question 1 :
In the following aqueous solutions<br>(A) 1 m sucrose <br>(B) 1 m potassium ferricyanide and <br>(C) 1 m potassium sulphate maximum value of vapour pressure of solution is that of :<br>
Question 2 :
Consider the figure and mark the correct option. <br><img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799bb7ced10523befee065' height='123' width='175' ><br>
Question 3 :
The main reason for the extremely low solubility of carbon dioxide in benzene $(C_{6}H_{6})$ at room temperature is due to which of the following?
Question 4 :
If a is the degree of dissociation of Na2SO<sub>4</sub>, the vant Hoff's factor (i) used for calculating the molecular mass is :<br>
Question 5 :
Correct combination among the following is <br>i) Water can be converted into vapour at ${ 100 }^{ 0 }C$ only<br>ii) Vapour pressure can be increased more than the atmospheric pressure at a certain temperature.<br>iii) Lowering of vapour pressure is directly proportional to mole fraction of solute.
Question 9 :
If $N_2$ gas bubbled through water at $ 293 K $ how many millimoles of $N_2$ gas would dissolve in 1 litre of water? Assume that exerts a partial pressure of $ 0.987 bar $. <div>Given that Henry's law constant for $N_2$ at $293 K$ is $76.48 K bar $ :</div>
Question 10 :
The boiling point of $C_{6}H_{6},\:CH_{3}OH,\:C_{6}H_{5}NH_{2}$ and $C_{6}H_{5}NO_{2}$ are $80\ ^{\circ}C$, $65\ ^{\circ}C$, $184\ ^{\circ}C$ $212\ ^{\circ}C$ respectively. <div>Which will show the highest vapour pressure at room temperature?</div>
Question 11 :
The vapour pressure of two pure liquids A and B, that form an ideal solution are $100$ and $900$ torr respectively at temperature T. This liquid solution of A and B is composed of $1$ mole of A and $1$ mole of B. What will be the pressure, when $1$ mole of mixture has been vapourized?
Question 12 :
$3$ moles of $P$ and $2$ moles of $Q$ are mixed, what will be their total vapour pressure in the solution if their partial vapour pressures are $80$ and $60$ torr respectively?
Question 13 :
Henry law constant for the solubility of methane in benzene at 298K is $4.27\times { 10 }^{ 5 }$ mm Hg then the solubility of methane in benzene at 298K under 760mm Hg is:<br/>
Question 14 :
$PbCl_2$ has maximum concentration of $1.0 \times 10^{-3}$ M in its saturated aq. solution at $25^0$C. Its solubility in $0.1$M NaCl solution will be :
Question 15 :
When gas is bubbled through water at $298K$, a very dilute solution of gas is obtained. Henry's law constant for the gas is $100kbar$. If gas exerts a pressure of $1$ bar, number of moles of gas dissolved in $1$ litre of water is:
Question 16 :
One having high vapour pressure at temperature below its melting point is?
Question 17 :
The values of the Henry's law constant of $Ar,CO_2,CH_4$ and $O_2$ in water at $25^0C$ are $40.30,1.67,0.41$ and $34.86\,kbar$, respectively. The order of their solubility in water at the same temperature and pressure is:
Question 18 :
The vapour pressure lowering caused by the addition of $100 \,g$ of sucrose (molecular mass = 342) to $1000 \,g$ of water if the vapour pressure of pure water at $25 ^\circ C$ is $23.8 \,mm \,Hg$
Question 19 :
When a liquid that is immiscible with water was steam distilled at $95.2^o$C at a total pressure of $99.652$ kPa, the distillate contained $1.27$ g of the liquid per gram of water. What will be the molar mass of the liquid if the vapour pressure of water is $85.140$ kPa at $95.2^o$C?<br/>
Question 20 :
Which of the following changes decreases the vapour pressure of water kept in a sealed vessel?