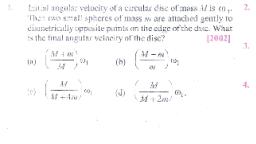Question Text
Question 1 :
Which of the statements about the reaction below are incorrect ?<br/>$2PbO(s) + C(s) \rightarrow 2Pb(s) + CO_2(g)$<br/><br/>(a) Lead is getting reduced.<br/>(b) Carbon dioxide is getting oxidised.<br/>(c) Carbon is getting oxidised.<br/>(d) Lead oxide is getting reduced
Question 2 :
Many grain products, including cereals and others that identify themselves as a 'source of iron'. List something called "reduced iron" among the ingredients.<br/>To which of the following would the term "reduced iron" most likely be referring?
Question 3 :
Identify the reducing agent in the redox reaction<br/><br/>$5SO^{2-}_3+ 2MnO^{-}_4 +6H^{+} \rightarrow 5SO^{2-}_4 +2Mn^{2+} +3H_2O$
Question 4 :
On heating blue coloured powder of copper(II) nitrate in a boiling tube copper oxide (black), oxygen gas and a brown gas X is formed. Which type of reaction is this?<br/>
Question 6 :
Which one of the following is present as an active ingredient in bleaching powder for bleaching action?
Question 7 :
$25ml, 0.2M\ Ca(OH)_{2}$ is neutralised by $10\ ml$ of $1M\ HCl$. The $pH$ of resulting solution is:
Question 8 :
The bleaching action of the bleaching powder is due to the liberation of 
Question 9 :
The pHof a soft drink is $3.82$. The hydrogen ion concentration wil be: (given antilog $0.18=1.5$)
Question 11 :
Where would you locate the element with electronic configuration 2,8 in the modem period table?
Question 12 :
The element having the least size from the following is :
Question 14 :
The nuclear charge actually experienced by an electron is termed as the effective nuclear charge. The effective nuclear charge Z* actually depends on the type of shell and orbital in which electron is actually present. The relative extent to which the various orbitals penetrate the electron clouds of other orbitals is $s>p>d>f$ (for the same value of $n$)<br/>The phenomenon in which penultimate shell electrons act as screen or shield in between nucleus and valence shell electrons and thereby reducing nuclear charge is known as shielding effect. The penultimate shell electrons repel the valence shell electron to keep them loosely held with the nucleus. It is thus evident that more is the shielding effect, lesser is the effective nuclear charge and lesser is the ionization energy.<br/>Which of the following is not concerned with effective nuclear charge?<br/>
Question 15 :
Assertion: The atomic size generally increases across a period and decreases down the group.
Reason: Atomic size depends upon valence shell electronic configuration.