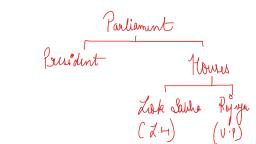
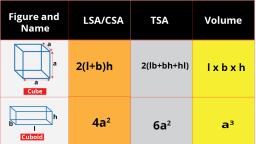
Question 1 :
The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
Question 2 :
Which of the following scientist identified the 'Concept of nucleus' in the structure of an atom ?
Question 3 :
Which of the following scientist identified the 'Concept of nucleus' in the structure of an atom ?
Question 4 :
The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Question 5 :
Which of the following scientist identified the 'Indivisibility of atoms' in the structure of an atom ?
Question 6 :
Which of the following correctly represent the electronic distribution in the Mg atom?
Question 7 :
In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed?
Question 8 :
An element X has a mass number 4 and atomic number 2. Write the valency of this element?
Question 9 :
Why did Rutherford select a gold foil in his $\alpha$–ray scattering experiment?
Question 11 :
An atom with 3 protons and 4 neutrons will have a valency of
Question 12 :
State true or false: Helium atom has 2 electrons in its valence shell but its valency is not 2.
Question 15 :
The ratio of the radii of hydrogen atom and its nucleus is ~ $10^{5}$. Assuming the atom and the nucleus to be spherical and if atom is represented by planet earth $‘R’=6.4\times 10^{8}m$ , estimate the size of the nucleus.
Question 16 :
Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the $\alpha$–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
Question 17 :
<img style='object-fit:contain' src='https://teachmint.storage.googleapis.com/question_assets/cbse_ncert/61b1d2b2f59b460d7261f60c.JPG' />
Find out the valency of atoms represented by the figure.
Question 18 :
Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the $\alpha$–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
Question 19 :
Rutherford’s α-particle scattering experiment led to the discovery of the ____________.
Question 20 :
An atom with 3 protons and 4 neutrons will have a valency of
Question 22 :
Which of the following is true for an element?
(i) Atomic number = number of protons + number of electrons
(ii) Mass number = number of protons + number of neutrons
(iii) Atomic mass = number of protons = number of neutrons
(iv) Atomic number = number of protons = number of electrons.
Question 24 :
The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
Question 25 :
Which of the following is true for an element?
(i) Atomic number = number of protons + number of electrons
(ii) Mass number = number of protons + number of neutrons
(iii) Atomic mass = number of protons = number of neutrons
(iv) Atomic number = number of protons = number of electrons.
Question 26 :
Dalton’s atomic theory successfully explained:
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportion
Question 27 :
One electron is present in the outer most shell of the atom of an element X. What would be the nature of charge on the ion formed if this electron is removed from the outermost shell ?
Question 28 :
Which of the following element has an atom with one electron, one proton and no neutron ?
Question 30 :
Why did Rutherford select a gold foil in his $\alpha$–ray scattering experiment?
Question 31 :
Rutherford’s 'alpha ($\alpha$) particles scattering experiment’ resulted in to discovery of _______.
Question 32 :
Dalton’s atomic theory successfully explained:
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportion
Question 33 :
Which of the following scientist identified the 'Neutron' in the structure of an atom ?
Question 34 :
Which of the following scientist identified the 'Canal rays' in the structure of an atom ?
Question 35 :
State true or false: In an atom the number of protons and electrons is always equal.
Question 36 :
Which of the following element has an atom with one electron, one proton and no neutron ?
Question 38 :
Helium atom has a valency of zero, even after having 2 electrons in its valence shell, because
Question 40 :
Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be ______ and _______ respectively.