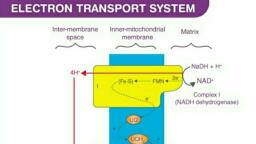
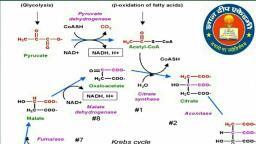
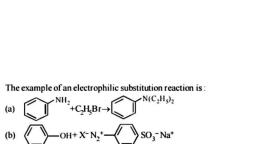
Question 1 :
0.52 g of dibasic acid required 100 mL of 0.1 <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fccdfdd8313cc8726705"> NaOH for complete neutralization. The equivalent weight of acid is:
Question 2 :
The formula which represents the simple ratio of atoms in a compound is called:
Question 3 :
A student performs a titration with different burettes and finds titre values of 25.2 mL, 25.25 mL, and 25.0mL. The number of significant figures in the average titre value is
Question 4 :
Stoichiometric ratio of sodium dihydrogen orthophosphate and sodium hydrogen orthophosphate required for synthesis of <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fed1a3d2442ab82937d8"> is
Question 5 :
If $0.01$ mole of solute is present in $500\ ml $ of solution, its molarity is:
Question 6 :
The number of mole present in 2 litre of 0.5 <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fcdcfdd8313cc872674e"> NaOH is:
Question 7 :
How many moles of sulfate ions are in $200 mL$ of a $2 M$ sodium sulfate solution?
Question 8 :
The weight, in grams, of KCl (Mol.wt. = 74.5) in 100ml of a 0.1M KCl solution is:
Question 9 :
<b></b>Calculate the volume in 10 millimoles of solute present in 0.08 M solution.
Question 10 :
Number of mole of 1 <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7fe38fdd8313cc8726aac"> gas at NTP are:
Question 11 :
Calculate the value of $100\ x$ if $x$ mL is the volume of water required to make $1\ \text N$ solution from $2$ mL concentration $HNO_3$.<br/>
Question 12 :
One mole, of $P_4O_{10}$ is allowed to react fully with dust and salt-free doubly distilled water and the volume is made up to 1L. What is the normality of the so-generated orthophosphoric acid?<br>$P_4O_{10} +6H_2O\rightarrow 4H_3PO_4$<br>
Question 13 :
An aqueous solution of glucose is 10% in strength. The volume in which 1 gram mole of it is dissolved will be:<br/>
Question 14 :
Calculate the molarity of a solution containing 20.7 g of potassium carbonate dissolved in 500 mL of solution (assume density of solution = 1 g mL$^{-1}$).
Question 15 :
The volume inline liters of $CO_{2}$ liberated at $STP$ when $10g$ of $90\%$ pure limestone is heated completely is:<br/>
Question 16 :
1.5 litre of solution of normality N and 2.5 litres of 2M HCl are mixed together. The resultant solution has a normality of 5. The value N is:
Question 17 :
Determine the molarity of solution obtained by mixing $50ml$ of $0.26M\ H_{2}SO_{4}$ solution with another $150ml$ of $0.48M\ H_{2}SO_{4}$ solution?
Question 18 :
In carbon disulphide $(CS_{4})$, the mass of sulphur in combination with $3.0\ g$ of carbon is
Question 19 :
The molarity of 15% (w/w) solution of ${ H }_{ 2 }{ SO }_{ 4 }$ of density 1.1 g/cc is:
Question 20 :
What is the normality of a $1 \,M$ solution of $H_3PO_4$?
Question 21 :
$1$ mole $N_{2}$ and $4$ mole $H_{2}$ are allowed to react in a vessel and after reaction $H_{2}O$ is added to the vessel. Aqueous solution required $1$ mole $HCl$. Mole fraction of $H_{2}$ in the gaseous mixture after the reaction is:
Question 22 :
A 3.4 g sample of H$_2$O$_2$ solution containing $x\%$ H$_2$O$_2$ by mass requires x ml of a KMnO$_4$ solution for complete oxidation under acidic condition. The molarity of KMnO$_4$ solution is :
Question 23 :
Two oleum samples $A$ and $B$ have labeling $109%$ and $118%$ respectively. $100\ g$ of $A$ is diluted to an a $1\ L$ solution and $100\ g$ of $B$ is diluted to a $1000\ mL$ solution. If both these solutions are mixed, then normality of $H_{2}SO_{4}$ (approx.) in final solution is:
Question 24 :
A mixture of $Na_2C_2O_4$ ($A$) and $KH_2C_2O_4.2H_2O$ ($B$) required equal volumes of $0.1$ M $KMnO_4$ and $0.1$ M $NaOH$ separately. Molar ratio of $A$ and $B$ in the mixture is :
Question 25 :
Molarity of $Ca_{3}(PO_{4})_{2}$ if the molarity of the calcium ions in the same solution is $3M$:
Question 26 :
$300$ g of an aqueous solution of a particular solute (containing $30$% solute by mass) is mixed with $400$ g of another aqueous solution of the same solute (containing $40$% solute by mass). In the final solution, mass $\%$ of solute is :<div>[Given, Molecular mass of solute $\displaystyle = 50$]<br/></div>
Question 27 :
$5.5\ mg$ of nitrogen gas dissolves in $180\ g$ of water at $273\ K$ and one atm pressure due to nitrogen gas. The mole fraction of nitrogen in $180\ g$ of water at $5\ atm$ nitrogen pressure is approximately :
Question 29 :
What is the mole fraction of the solute in a $1.00$ m aqueous solution?
Question 30 :
A solution of $KMnO_4$ containing $3$ g/L is titrated with a solution of $H_2O_2$ containing $2$ g/L.<div>The volume (in ml) of $KMnO_4$ required to react with $20$ mL $H_2O_2$ solution is: (as nearest integer) </div>