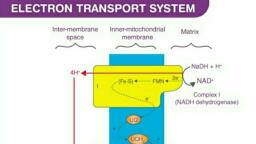
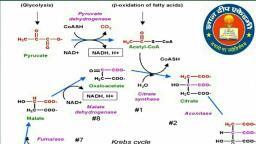
Question 1 :
If r is radius of first orbit, the radius of nth orbit of the H atom will be :
Question 2 :
The total energy of the electron revolving around the nucleus is:
Question 3 :
<div>State True or False.<br/></div>The velocity of the electron is maximum in the Bohr's last orbit.
Question 4 :
Energy of the electron in hydrogen in its first orbit is:
Question 6 :
Out of given atoms which atoms has highest energy of 2s-subshell ?
Question 7 :
The maximum possible values of magnetic orbital quantum number ($m_{l})$ are
Question 9 :
Which of the following parameters are same for all hydrogen like atoms and ions in their ground state?
Question 10 :
The ionisation energy for excited hydrogen atom in eV will be :
Question 11 :
Which of the following electron transition in hydrogen atom will require the largest amount of energy?
Question 13 :
The possible subshells in $n = 3$ energy shell are :
Question 14 :
<div>State whether the given statement is true or false:</div><br/>With the increase in distance from the nucleus, energy of electron increases and the velocity of electron decreases.<br/>
Question 15 :
The energy of second orbit of hydrogen is equal to the energy of:
Question 16 :
The velocity of electron in third excited state of $\displaystyle Be^{3+}$ will be:
Question 17 :
According to de-Broglie wavelength for electron in an orbit of hydrogen atom is $10^{-9}\ m$. The principle quantum number for this electron is
Question 18 :
The ratio of velocity of the electron in the third and fifth orbit of $Li^{2+}$ would be:
Question 19 :
By what percent does the distance between successive orbits of 1 and 2 to 2 and 3 of hydrogen atom vary?
Question 20 :
In a hydrogen atom, an electron jumps from the third orbit to the first orbit. Find out the frequency of the spectral line. $\left( { R }_{ H }=1.09678\times { 10 }^{ 7 }{ m }^{ -1 } \right) $.
Question 21 :
The energy of an electron in the $3s$ orbital (excited state) of H-atom is: 
Question 22 :
The velocity of electron in second shell of hydrogen atom is :<br/>
Question 25 :
Not considering the electron spin, the degeneracy of second excited state is 9, while the generality of the first excited state of $H$ atom is: 
Question 26 :
In an atom the shell which has a maximum two electrons is:
Question 27 :
<div>A formula analogous to the Rydberg formula applies to the series of spectral lines which arise from transitions from higher energy level to the lower energy level of hydrogen atom.<br/>A muonic hydrogen atom is like a hydrogen atom in which the electron is replaced by a heavier particle, the 'muon'. The mass of the muon is about $207$ times the mass of an electron, while the charge remains same as that of the electron. Rydberg formula for hydrogen atom is:<br/>$\dfrac { 1 }{ \lambda  } ={ R }_{ H }\left[ \dfrac { 1 }{ { n }_{ 1 }^{ 2 } } -\dfrac { 1 }{ { n }_{ 2 }^{ 2 } }  \right] \left( { R }_{ H }=109678{ cm }^{ -1 } \right) $<br/></div>Radius of first Bohr orbit of muonic hydrogen atom is
Question 28 :
The ratio of the momentum of a proton and a $\alpha $ - particle which is accelerated from rest by a potential difference of 200 V. $m_{p}$ and $m_{\alpha }$ are masses of the proton and ${\alpha }$- particles: 
Question 29 :
As an electron is brought from an infinite distance close of nucleus of the atom, the energy of electron:
Question 30 :
The angular speed $(\omega)$ of an electron revolving in $n^{th}$ Bohr orbit and corresponding principal quantum number(n) are related as ____________.
Question 31 :
The ratio of the difference in energy between the first and second Bohr orbits to between the second and third Bohr orbit is:
Question 33 :
The energy of the second Bohr orbit in the hydrogen atom is $-3.41\ eV$. The energy of the second Bohr orbits of $He^+$ ion would be:
Question 34 :
According to the Bohr model of the atom, which electron transition will emit the lowest energy photon?
Question 36 :
The two electrons have the following sets of quantum numbers.<br/>X: 3, 2, -2, +1/2<br/>Y: 3, 0, 0, + 1/2<br/>What is true of the following?
Question 37 :
The energy of the electron in the second and third Bohr orbits of the hydrogen atom is -5.42 $\times$ 10$^{-12}$ erg and -2.41 $\times$ 10$^{-12}$ erg, respectively. The wave length of the emitted radiation when the electron drops from third to second orbit will be :
Question 39 :
Consider a hypothetical hydrogen like atom. The wavelength in $A^o$ for the spectral lines for transition from $n=p$ to $n=1$ are given by:<br/>$\lambda =\displaystyle\frac{1500p^2}{p^2-1},$ where $p > 1$<br/>Find the ionization potential of this element?<br/>
Question 41 :
If the ionization energy for hydrogen atom is $13.6 eV$, then the ionization energy for ${He}^{+}$ ion should be:
Question 42 :
Among 4p, 4s, and 3d orbitals, 3d orbital has the least energy.
Question 44 :
If the ionization energy of hydrogen is $313.8 \; {Kcal}/{mol}$, then the energy of electron in ${2}^{nd}$ excited state will be:
Question 45 :
Calculate the radius of Bohr's first orbit for hydrogen atom and the energy of electron in this orbit:
Question 46 :
<div>For $H$-like atoms :</div><div>      </div><div>            $\displaystyle E_n=-\frac{Z^2Rh}{n^2};u_n=\frac{u_1Z}{n}$ and $r_n=\frac{r_1\times n^2}{Z};$ where $Rh$ is Rydberg.<br/></div><br/>What is the potential energy of electron in $2^{nd}$ orbit of $H$-atom?<br/>
Question 47 :
Number of waves made by the pion when orbiting in third excitation state are
Question 49 :
What is the energy required to move the electron from the ground state of H atom to the first excited state? Given that the ground state energy of H atom is 13.6 eV and that the energy E$_n$ of an electron in n$^{th}$ orbital of an atom or ion of atomic number Z is, given by the equation $E_n =(13.6Z^2/n^2)$
Question 50 :
If elements of quantum number greater than $'n'$ were not allowed, the number of possible elements in nature would have been
Question 51 :
Calculate the ratio of energies of $2^{nd}$ orbits of hydrogen, $He^+, Li^{+2}$.<br/>
Question 52 :
Electrons accelerated by potential V are diffracted from a crystal. If $\mathrm{d}= 1\mathrm{A}$ and $\mathrm{i}=30^{0},\ \mathrm{V}$ should be about: <div>[$\mathrm{h}=6.6\times 10^{-34}$ Js, $\mathrm{m}_{\mathrm{e}}=9.1\times 10^{-31}$ kg, $\mathrm{e}=1.6\times 10^{-19}\mathrm{C}$]<br/></div>
Question 53 :
The total energy of a hydrogen atom in its ground state is $-13.6\ eV$. If the potential energy in the first excited state is taken as zero then the total energy in the ground state will be:
Question 54 :
The number of revolutions of an electron in the second Bohr orbit in one second is:<br>
Question 55 :
The ionization energy of hydrogen atom is $13.6$ eV. The longest wavelength of hydrogen spectrum in the ultraviolet region is expected to be:
Question 56 :
Assertion: 3s, 3p and 3d subshells of hydrogen have the same energy. <br/>Reason: Energy of subshells in the hydrogen atom, depends on the principal quantum number (n) and azimuthal quantum number (l). <br/>
Question 57 :
The energy of the second Bohr orbit in the hydrogen atom is $-3.41 \,eV.$ The energy of the second Bohr orbit of $He^{+}$ ion would be:
Question 58 :
A hydrogen-like atom (atomic number $Z$) is in a higher excited state of the quantum number $n$. This excited atom can make a transition to the first excited state by successfully emitted to photons of energies $10.20eV$ and $17.00eV$ respectively.<div>       Alternatively, the atom from the same excited state can make a transition to the second excites state by the successively emitting two photons of energy $4.25ev$ and $5.95ev$ respectively. Determine the values of $n$ and $Z$ (ionization energy of hydrogen atom $=13.6eV$).<br/></div>
Question 60 :
If the wavelength of the photon emitted from an electron jump n $=$ 4 to n $=$ 2 in a H-like species is 1216 $\overset{o}{A}$, then the species is :
Question 61 :
Consider the hydrogen atom to be a proton embedded in a cavity of radius $a_0$ (Bohr's radius), whose charge is neutralized by the addition of an electron to the cavity in vacuum, infinitely slowly. Then the wavelength of the electron when it is at a distance of $a_0$ from the proton will be <br/>
Question 62 :
The ratio of speed of electron in $I$ orbit of $H$-atom to $IV$ orbit of $He^+$ ion is :<br/>
Question 63 :
The ratio of ground state energy of $Li^{2+}, He^+$ and H is :
Question 64 :
The wavelength($\lambda_n$) of the pion orbiting in nth stationary state is given by
Question 65 :
Check the correctness of the following statements about the Bohr Model of hydrogen atom:<br>$(i)$ The acceleration of the electron in $n=2$ orbit is more than that in $n=1$ orbit.<br>$(ii)$ The angular momentum of the electron in $n=2$ orbit is more than that in $n=1$ orbit.<br>$(iii)$ The KE of the electron in $n=2$ orbit is less than that in $n=1$ orbit