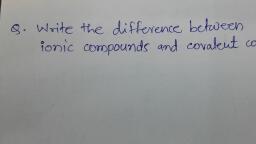Question 1 :
All bond angles are exactly equal to <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c9376f3020298ca137a9"> in
Question 3 :
Which of the following compounds has dipole moment approximately equal to that of chlorobenzene?
Question 6 :
Oxygen and sulphur both are the member of same group in Periodic Table but <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c8e5399925718ac6ab8b"> is liquid while <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c92a6f3020298ca1378a"> is gas because
Question 8 :
Assertion: Ionic compounds are bad conductors of electricity.
Reason: In ionic compounds mobile ions exists.
Question 9 :
The structure of glycine (amino acid) is $H_{2}N-CH_{2}COO^{-}$ ($z$ witter ion). Select the correct statement:-
Question 12 :
The bond formed between the two atoms by mutual sharing of electrons so as to complete their octets or duplets gives :<br/>
Question 13 :
Atoms or group of atoms which are electrically charged are known as:
Question 20 :
The value of bond order in nitrogen and oxygen molecule is:
Question 22 :
The bond order of <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c8e9c2a2ae2953d94236"> molecule on the basis of molecular orbital theory is:
Question 23 :
In a crystal, the atoms are located at the positions of:
Question 27 :
Formal charge at oxygen atom I, II and III<br><img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ef6d7d9180da34ba6ee438f"><br>
Question 30 :
One would expect the elemental form of <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c942ab3481716f4b70f9"> at room temperature to be:
Question 31 :
Some of the properties of the two species, <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c95b6f3020298ca137da"> and <img style='object-fit:contain' src="https://storage.googleapis.com/teachmint/question_assets/NEET/5ea7c9036f3020298ca1372a"> are described below. Which one of them is correct?
Question 33 :
Consider the given organometallic compound.<br>(I)$(CH_{3})_{2}\ Hg$ (II) $(CH_{3})_{2}\ Zn $ (III)$(CH_{3})_{2}\ Mg$ (IV)$CH_{3}Li$<br>The correct decreasing order of ionic character is :<br>
Question 35 :
Which of the following pairs of ions is isoelectronic and isostructural?
Question 36 :
Which of the following molecule possesses polar and nonpolar covalent bond ?
Question 40 :
Among the following mixtures, dipole dipole as the major interaction, is present in