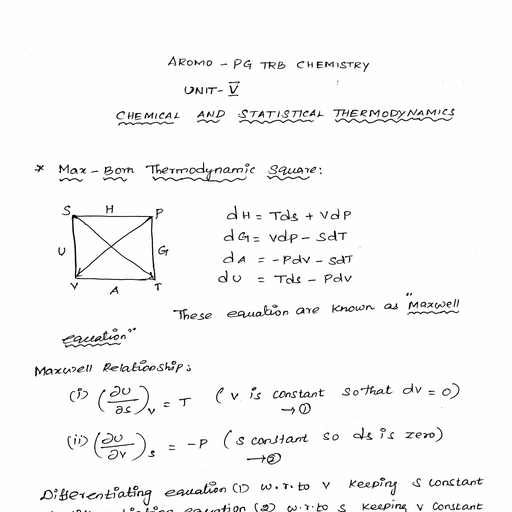
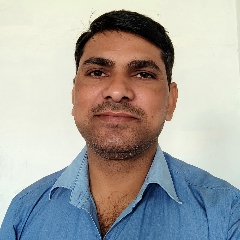
Question 1 :
For reaction A + 2B <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799d55e89a3d214216ffcc' height='21' width='36' >C + D rate law R = k[A]<sup>1</sup>[B]<sup>2</sup>. By what factor would the rate changes if concentration of A is doubled & that of B is halved ?<br>
Question 2 :
The rate of the reaction, 3A + 2B → Products is given by the rate expression :Rate = k[A][B]<sup>2</sup> .If A is taken in excess, the order of the reaction would be:<br>
Question 3 :
The rate of a gaseous reaction is halved when the volume of the vessel is doubled. The order of reaction is<br>
Question 4 :
The rate equation for the reaction 2A + B <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799dbde89a3d2142170165' height='21' width='43' > C is found to be: rate = k[A] [B]. The correct statement in relation to this reaction is that the :<br>
Question 5 :
In the respect of the equation k = Ae<sup>-Ea/RT</sup> in chemical kinetics, which one of the following statements is correct :<br>
Question 6 :
A certain zero order reaction has k = 0.025 M s<sup>-1</sup> for the disappearance of A. What will be the concentration of A after 15 seconds if the initial concentration is 0.5 M?<br>
Question 7 :
Gaseous N<sub>2</sub>O<sub>5 </sub>decomposes according to the following equation :<br>N<sub>2</sub>O<sub>5</sub>(g) → 2NO<sub>2</sub>(g) + <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799d9be89a3d2142170115' height='36' width='15' > O<sub>2</sub>(g)<br>The experimental rate law is -Δ[N<sub>2</sub>O<sub>5</sub>]/Δt = k[N<sub>2</sub>O<sub>5</sub>]. At a certain temperature the rate constant is k = 5.0 x 10<sup>-4</sup>/sec. In how seconds will the concentration of N<sub>2</sub>O<sub>5</sub> decrease to one-tenth of its initial value?<br>
Question 8 :
The half - life of a radioisotope is four hours. If the initial mass of the isotope was 200 g, the mass remaining after 24 hours undecayed is :<br>
Question 9 :
The activation energy of a reaction is zero. The rate constant of the reaction -<br>
Question 10 :
The order of a reaction can be predicted with the help of ______________.<br/>
Question 11 :
For the reaction <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799dfbe89a3d21421701f3' height='29' width='98' > rate is given by R = [A][B]<sup>2</sup> than the order of the reaction is
Question 12 :
For a chemical reaction X ―→ Y, the rate of reaction increases by a factor 1.837 when the concentration of X is increased by 1.5 times. The order of the reaction with respect to X is<br>
Question 13 :
Activation energy of a chemical reaction can be determined by -<br>
Question 14 :
For the decomposition of HI at 1000 K (2HI → H<sub>2</sub> + I<sub>2</sub>), the following data were obtained<table><tr><td> [HI] M</td><td>Rate of decomposition of HI (mol L<sup>-1</sup>s<sup>-1</sup>)</td></tr><tr><td>0.1</td><td> 2.75 × 10<sup>-8</sup></td></tr><tr><td>0.2</td><td> 11 × 10<sup>-8</sup></td></td></tr><tr><td>0.3</td><td> 24.75 × 10<sup>-8</sup></td></tr></table>The order of reaction is :<br>
Question 15 :
The order and molecularity of the chain reaction, ${ H }_{ 2 }\left( g \right) +{ Cl }_{ 2 }\left( g \right) \xrightarrow [ ]{ \quad hv\quad } 2HCl\left( g \right) $, are:
Question 16 :
Which of the following will result when the temperature is increased as the chemical reaction proceeds?<br>I. Increased molecular collision frequency<br>II. Increased numbers of molecules possess energy greater than the activation energy<br>III. Decreased randomness of the system
Question 17 :
A reaction was found to be second order with respect to the concentration of carbon monoxide. If the concentration of carbon monoxide is doubled, with everything else kept the same, the rate of reaction will be
Question 18 :
The minus sign in rate <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799cfcced10523befee218' height='41' width='51' >indicates the _____ in concentration of the _____with time. The rate of a reaction is always _____ quantity. The rate of reaction increases with ______ in concentration of reactants, the blanks in the questions corresponds to<br>
Question 19 :
The rate constant of a first order reaction is 10<sup>-3</sup> min<sup>-1</sup> at 300 K. The temperature coefficient of the reaction is 2. What is the rate constant of the reaction at 350 K approximately ?<br>
Question 20 :
For N<sub>2</sub>(g) + 3H<sub>2</sub>(g) → 2NH<sub>3</sub>(g) + 22 kcal, E<sub>a</sub> for the reaction is 70 kcal. Hence, the activation energy for <br>2NH<sub>3</sub>(g) → N<sub>2</sub>(g) + 3H<sub>2</sub>(g) is<br>
Question 21 :
The first order rate constant for the decomposition of <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799d6ae89a3d2142170066' height='20' width='36' >is <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799d6aced10523befee4d4' height='19' width='89' >. The half-life period for this decomposition in seconds is<br>
Question 22 :
In the following reaction, how is the rate of appearance of the underlined product related to the rate of disappearance of the underlined reactant?<br>$BrO_{3(aq)}^{-}+\underline {5Br^-}_{(aq)}+6H^+_{(aq)}\rightarrow \underline {3Br_{2(1)}}+3H_2O_{(1)}$
Question 23 :
Activation energy of a chemical reaction can be determined by ________<br>
Question 24 :
The experimental data for the reaction,<br>2A + B<sub>2</sub>→ 2AB, is <br><table><tr><td>Expt.</td><td> [A]</td><td> [B<sub>2</sub>]</td><td> Rate (s<sup>-1</sup>)</td></tr><tr><td>1.</td><td> 0.50</td><td> 0.50</td><td> 1.6×10<sup>-4</sup></td></tr><tr><td>2.</td><td> 0.50</td><td> 1.00</td><td> 3.2× 10<sup>-4</sup></td></tr><tr><td>3.</td><td> 1.0</td><td> 1.00</td><td> 3.2×10<sup>-4</sup></td></tr></table>The rate equation for the above data is -<br>
Question 25 :
For the reaction A + 2B → C, rate is given by R = [A] [B]2 then the order of the reaction is :<br>
Question 26 :
Rate of a reaction can be expressed by Arrhenius equation as <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799e0cced10523befee680' height='29' width='105' >In the equation, E represents<br>
Question 27 :
The rate constants of a reaction at 300K & 280K respectively are $K_1 $&$K_2$. Then:
Question 28 :
The rate constant of a reaction is found to be 3× 10<sup>-3</sup>mol L<sup>-1</sup> min<sup>-1</sup>. The order of the reaction is<br>
Question 29 :
The value rate constant of a pseudo first order reaction __________.<br>
Question 30 :
If the rate expression for a reaction is $\dfrac { dx }{ dt } =k{ \left[ A \right] }^{ { 1 }/{ 2 } }{ \left[ B \right] }^{ { 3 }/{ 2 } }$, the overall order of the reaction is:
Question 31 :
A reaction was found to be second order with respect to the concentration of carbon monoxide. If the concentration of carbon monoxide is doubled, with everything else kept the same, the rate rate of reaction will<br>
Question 32 :
A reaction was found to be second order with respect to the concentration of carbon monoxide. If the concentration of carbon monoxide is doubled, with everything else kept the same, the rate of reaction will be<br>
Question 33 :
The value of rate of pseudo first order reaction depends upon<br>
Question 34 :
For a reversible reaction, A + B <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799cfaa2bddf222e0af3ab' height='16' width='11' > C+ D, the graph for rate of reaction with time is given below.<br>Mark the terms (p), (q) and (r).<br><img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799cfae89a3d214216fd98' height='105' width='107' ><br>
Question 35 :
The following mechanism has been proposed for the reaction of NO with Br<sub>2</sub> to from NOBr<sub>2</sub><br>NO(g) + Br<sub>2</sub>(g) <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799db2e89a3d214217014b' height='15' width='42' > NOBr<sub>2</sub>(g)<br>NOBr<sub>2</sub>(g) + NO(g) ―→ 2NOBr(g)<br>If the second step is the rate determining step, the order of the reaction with respect to NO(g) is<br>
Question 36 :
For the reaction system 2NO (g) + O<sub>2</sub>(g) ―→ 2NO<sub>2</sub>(g) Volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O<sub>2</sub> and second order with respect to NO, the rate of reaction will -<br>
Question 37 :
75% of a first order reaction was completed in 32 minute, when was 50% of the reaction completed?<br>
Question 38 :
For a reaction A + B → C + D, if the concentration of A is doubled without altering the concentration of B, the rate gets doubled. If the concentration of B is increased by nine times without altering the concentration of A, the rate gets tripled. The order of the reaction is:<br>
Question 39 :
A zero order reaction is one in which the rate of the reaction is independent of ______________.<br/>
Question 40 :
The energies of activation for forward and reverse reactions for A2 + B<sub>2</sub><img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799d58ced10523befee45a' height='18' width='39' > 2AB are 180 kJ mol<sup>-1</sup> and 200 kJ mol<sup>-1</sup> respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol<sup>-1</sup>. The enthalpy change of the reaction (A2 + B<sub>2</sub>→ 2AB) in the presence of catalyst will be (in kJ mol<sup>-1</sup>).<br>
Question 41 :
The gaseous reaction A (g) ―→ 2B (g) + C (g) is found to be first order with respect to A. If the reaction is started with p<sub>A</sub> = 90 torr the pressure after 10 min is found to be 180 torr. The rate constant of the reaction is<br>
Question 42 :
The rate constant is numerically the same for three reactions of first, second and third order respectively. Which of the following is correct : (A = reactant) -<br>
Question 43 :
The rate of a chemical reaction doubles for every 10<sup>o</sup>C rise of temperature. If the temperature is raised by 50<sup>o</sup>C, the rate of the reaction increases by about :
Question 44 :
The rate of a reaction increased by 2.5 times when the temperature is raised from 300 K to 310 K. If k is the rate constant at 300 K, then the rate constant at 310 K will be equal to -<br>
Question 46 :
In a first order reaction, the concentration of the reactant decreases from 0.8 M to 0.4 M in 15 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M is :<br>
Question 47 :
For the reaction 2NO<sub>2</sub> + F<sub>2 </sub>―→ 2NO<sub>2</sub>F, <br>following mechanism has been provided:<br>NO<sub>2</sub> + F<sub>2</sub><img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799da1ced10523befee58e' height='21' width='51' >NO<sub>2</sub>F + F <br>NO<sub>2</sub> + F <img style='object-fit:contain' src='https://storage.googleapis.com/teachmint/question_assets/JEE%20Main/5e799da1a2bddf222e0af72a' height='21' width='47' >NO<sub>2</sub>F<br>Thus rate expression of the above reaction can be written as -<br>
Question 48 :
The rate constant K<sub>1</sub> of a reaction is found to be doubles that of rate constant K<sub>2</sub> of another reaction. The relationship between corresponding activation energies of the two reactions at same temperature (E1 and E2) can be represented as :<br>
Question 50 :
Two reactions A ―→ products and B ―→ products have rate constant, k<sub>a</sub> and k<sub>b</sub> respectively at temperature T and activation energies are E<sub>a</sub> and E<sub>b</sub> respectively. If k<sub>a</sub>> k<sub>b</sub> and E<sub>a</sub><E<sub>b</sub> and assuming the frequency factor A in both the reactions are same then<br>