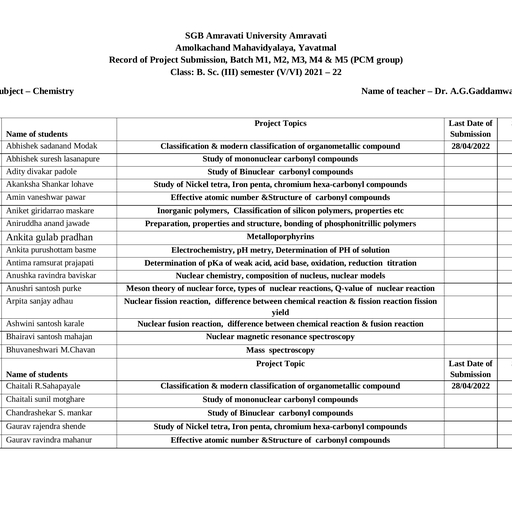
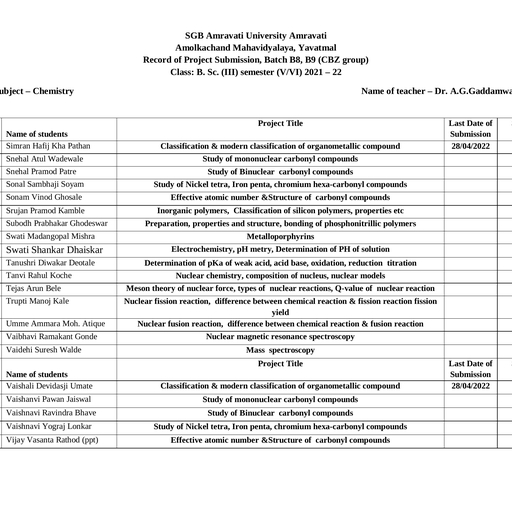
Page 1 :
VIVA — VOCE QUESTIONS, Physical Chemistry Experiments, , 1. Define electrolytic conductivity? ., Ans. It is ability of a solution to carry current., . 2. How current in solutions is carried out?, Ans. Current in solutions is carried out by migration of ions., 3. Cite factors on which rate of migration of ions depends?, , Ans. The rate of migration of ions depends on charge of ion, size, |, microscopic viscosity of the medium and magnitude of potential, , gradient., 4. Define ionic mobility?, Ans. _ Itis velocity of ion in the solution., 5: What is conductance? Mention its SI unit?, Ans. _ It is reciprocal of resistance. SI unit of conductance is S., 6. What is SI unit of specific conductance?, , Ans. SI unit of conductance is Sm"(Siemens per meter.), , Scanned with CamSce
Page 2 :
A Text Book of Practical Chemistry, , 27, , What is the effect of temperature o}, Conductance increases by 2-3%for, Define specific conductance/, , It is conductance of cube of a soluti, parallel plates] cm? and 1 cm apart., Define equivalent conductance?, , The conductance of a solution conta, electrolyte., , Define molecular conductance?, , The conductance ofa solution containin, What is conductivity water?, , Nn conductance of solution?, per degree rise of temperature,, , on contained between two, ining 1 g equivalent of an, , 8 1 g mole of an electrolyte., , A distilled water having Specific conductance 1x10 Scm" or less, is called as conductivity water., , What is the effect of dilution on specific , equivalent and molecular, conductance?, , Specific conductance decreases where as equivalent and molecular, conductance increases on dilution., , What are different types of conductometric titrations?, , a) Acid-base titrations, b) Replacement titration c) Precipitation, titrations d) Complexometric titrations,, , Why direct current is not used in conductometric measurements?, , If direct current is used, some electrolytic reactions take at both, the electrodes. Hence correct value of conductance will not be, obtained., , What is an electrochemical cell?, , A device in which chemical energy is converted into electrical energy., What is an electrolytic cell?, , A device in which electrical energy is converted into chemical energy., What is electrode potential?, , The potential developed on an electrode due to formation of an, , electrical double layer at the interface of an electrode and electrolyte, , is known as electrode potential., , Is it possible to determine single electrode potential?, , No, it is possible to determine single electrode potential., , OCdINICU WILT Udll ISCé
Page 3 :
Part Ill (Sem vg a, = Sem vi |, , , , 28, . . 2, , 19. What is aie mixture of quinine and hydroquinone., , a What are different types of potentiometric titrations?, , As a) Acid-base b) Redox c) Precipitation and d) Complex ometrig, titrations. ©, , 21. What is an indicator electrode?, , Ans. Anelectrode is one whose potential changes as a result of change, in concentration of ions with which it is reversible., , 22. What is meant by standard solution?, , Ans. Asolution whose concentration is exactly known., , 23. Whatis primary standard solution?, , Ans. A solution which is used as a reference to determine the , concentration of other solutions., 24. What are the criterion for a substance to be used as a primary, , standard substance ?, , Ans. Thesubstance should be available in pure state, readily soluble in, water, non hygroscopic and should be stable on storage. {, , 25. Can NaOH be used as primary standard substance?, , Ans. No, it can not be used since it is hygroscopic., , 26. Acetic acid is a weak acid?, , Ans. — Because it ionizes slightly in aqueous solution., , 27. Define plane polarized light?, , Ans. Light which vibrates only in one plane is called plane polarized, light., , 28. What are the factors that affect angle of rotation?, , Ans, a) nature of substance, b) nature of solvent c) wavelength, 4), , temperature e) thickness of liquid column., 29. What is the nature of optical activity?, Ans. It is constitutive Property., , 30. Which compounds are optically active?, Ans. The organic compounds having at least one asymmetric carbon, atom., Re Ke, , Ovaimieu wit GaMSce