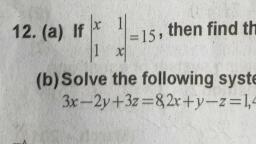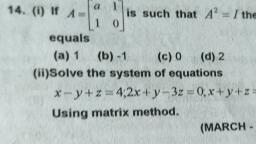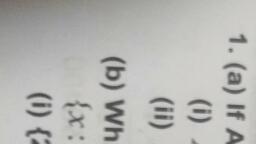Page 1 :
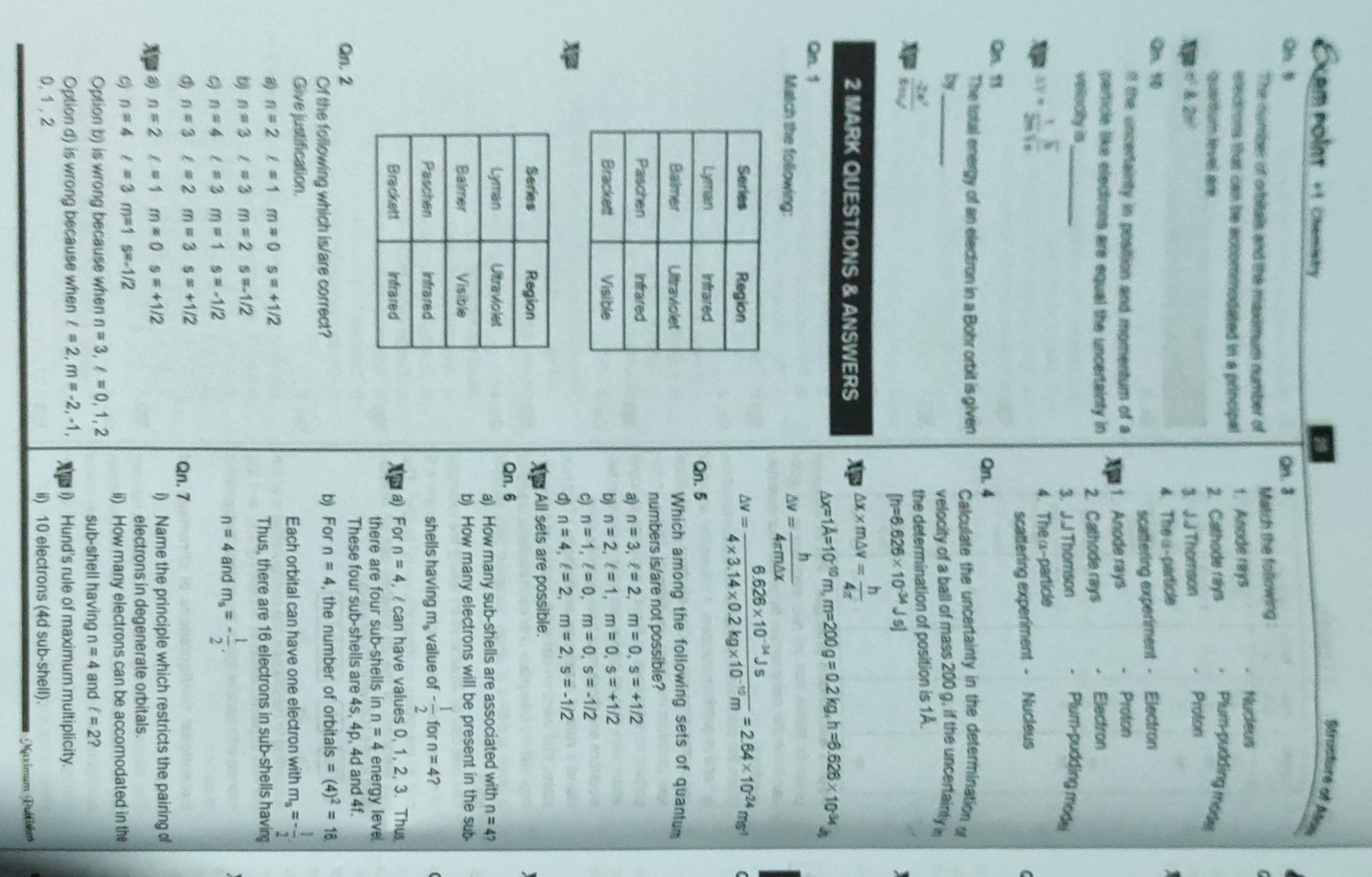
ae ons a ‘, Ther octet ate rhe mater of | Match ine Fone =, enn ntsenatennmestones 1. Anaee 1099 4, uatur ee ae 2 Cathode (ays, , er te 3. 3.4 Thomson, , Ga 4 Thea-patice, , SIN ccerigety i pOSmOn and momentum of 9, 1 Anode rays, ONE UE COCO Ae equa! the uncertenty in, tenia 2 Cathode rays, ee, “ = oe 4 The «-particle, Ca, , alae eaa ate Calculate the uncertainty in the det, , oe" the determination of position is 1A., we Bray, , , , , , ae 1A=10m, m=200g = 0.2 kg, 16.628 x 1¢ \, , h ;, ene , fem a |, 662610 "Js 7, , “i Aantal? a c, , , , Qn. 5, Which among the following sets of c, numbers is/are not possible?, a n=3, £22, m=0, s= 41/2, By n=2, f=1, m=0,5=+1/2, c) n=1, /=0, m=0, s=-1/2, @ n=4, f=2, m=2,s=-1/2, Nip All sets are possible., Qn. 6 i, a) How many sub-shells are associated with n=4, b) How many electrons will be present in the sug, shells having m, value of ~~ for n= 4? ™, Xp 4) Forn = 4, /can have values 0, 1, 2, 3, there are four sub-shells in n = 4 energy lev¢, Qn. 2 These four sub-shells are 4s, 4p, 4d and 4f. —, , Of the following which is/are correct? b) For n = 4, the number of orbitals = (4)? = 1, , are je a ' Each orbital can have one electron with m, =, = =, , Hes Ce Wed En eye Thus, there are 16 electrons in sub-shelis, , ones £83 m=2 $*1/2, , , , , , I, Qn=4 (23 m1 9*-12 n= 4nd m,* *;. :, Gono 482 m=3 g#+1l2 Qn.7 j, Mp 4 n=2 481 m*0 #412 )) Name the principle which restricts the pairings, , : electrons in degenerate orbitals., G nes 4 #3 m*1 g*-122 il) How many electrons can be accomodated int, Orfion 0) is wong because when n= 3, / =0,1,2 sub-shell having n= 4 and (= 2?, , Option 4) is wrong because when / *2,m*-2,-1, Nye) Hund’s rule of maximum multiplicity., , 6,1,2 i) 10 electrons (4d sub-shell). 4, mam Myxer i