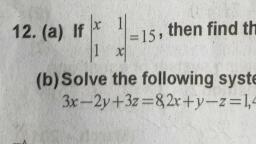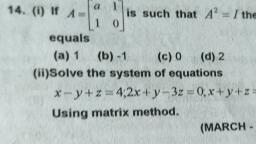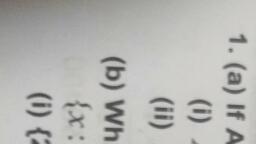Page 1 :
MEERUT *! Chemistry, , yn. 11, , A student argued that the 3d orbitals will be filled, , only after the 4s orbital is completely filled in, , accordance with aufbau principle. Then another, student opposed by saying that it is not true for certain, elements like Cr and Cu., , a) Whose argument is correct?, , b) Write electronic configurations of 24Cr and >9Cu., Justify your answer,, , a) Both arguments are correct., , b) CrandCu have anomalous electronic configurations., This is because half filled and completely filled, sub-shells have extra stability due to the symmetrical distribution of electrons and maximum, exchange energy., 24Cr = 18? 2s? 2p® 35? 3p® 3d°4s' OR [Ar]3d54s', This is due to the extra stability of half filled 3q5, sub-shell., 2aCu = 18% 2s? 2p® 3s? 3p§ 3q19 451 OR, [Ar]3d"°4s' This is due to the extra stability of, completely filled 3d'° sub-shell., , Qn. 12, a) What are the atomic numbers of elements whose, outermost electronic configurations are given by, i) 3s! ii) 3p%?, b) Which of the following are isoelectronic species?, Na*, K*, Mg**, Ca2*, S?- Ar, c) What will be the wavelength of a ball of mass, 0.1 kg moving with a velocity of 10 ms"?, NYS 8) i) 3s'- Atomic number is 11 (Na), ii) 3p° - Atomic number is 17 (Cl), b) Na*, Mg** (both of them have same no. of, electrons, i.e., 10 each), , _h _ 6.626x10“Js, , 34m, mv 0.4 kgx10 ms" =6.626x10, , Qn. 13, Quantum numbers are a set of four numbers used to, designate electron in an atom., i) How many electrons in an atom can have the, following quantum numbers, n= 1, 2=0?, ii) Give the quantum numbers of the valence electron, of an atom with atomic number 13., iii) Draw the shape of orbital having n= 1 and 4=0., NW!) 2 electrons (i.e., 1s orbital), ii) The element with atomic number 13 is aluminium., 13Al > [Ne] 3s?3p', The valence electron is in the 3p orbital. Hence,, the quantum numbers for the valence electron are, n= 3, €=1,m=-1,0,+1, ili) It is the 1s orbital., , Structure of Atom, Qn. 14, a) i) What is meant by line spectra or atomic, Spectra?, , il) Name the series of lines in the hydrogen, Spectrum belonging to the visible region., , li) What is the wave length of light emitted, when the electron in a hydrogen atom, undergoes transmission from n = 4 to, n= 2? (Ry = 109677 cm”), , b) State the principles/rules for filling of orbitals in, atoms., , XW a) |) Line spectra or atomic spectra are the spectra obtained from excited atoms due to emission of radiation. The emitted radiation is identified by the appearance of bright lines., , ii) Balmer series, , iil) Ny=2, no=4, , , , , , ae 10967| 5 — zen", , = 109677 eA, 4 16, , , , cm~' = 109677 xem", , = 20564.44 cm", , 1, ~ 20564.44 cm", , Aufbau principle: \n the ground state of the, atoms, the orbitals are filled in order of their, increasing energies., , il) Pauli’s exclusion principle: No two electrons, in an atom can have the same set of four, quantum numbers., , iii) Hund’s rule of maximum multiplicity: Pairing, of electrons in the orbitals belonging to the, same subshell does not take place until each, orbital belonging to that subsehlil has got one, electron each i.e., it is singly occupied., , = 4.863 10-°cm, +) a), , Qn. 15, , Line emission spectra are often called finger print of, , atoms., , a) Justify the above statement., , b) Yellow light emitted from a sodium lamp has a, wave length (A) of 580 nm. Calculate the frequency, and wave number of this yellow light., , Ny 2) Each element has a unique line emission spectrum. The characteristic lines in atomic spectra, can be used in chemical analysis to identify unknown atoms in the same way as finger prints, are used to identify people., , c 3x10°*ms”’, , = SAS R710" He, rE SOO, 1 1, $= 55 EX Om, °X 580x10m, , Meximum Publishers