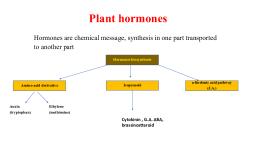
Page 1 :
PLANT TISSUE CULTURE, Definition: Plant tissue culture is the in-vitro cultivation of plant cell or tissue under, aseptic and controlled environmental conditions in liquid or on semisolid well, defined nutrient medium for the production of primary and secondary metabolites or to, regenerate plant, The whole process require a well-defined culture laboratory and nutrient medium, Totipotency: It is the ability of the plant cell to grow and divide, Cell Totipotency:, It is the potentiality of plant cell to regenerate the whole organism from a single cell, Totipotent cells from plants have been used in tissue-culture techniques to, produce improved plant materials that are pathogen-free and disease-resistant, The cells are obtained from stem, root or other plant parts and are allowed to grow, in culture medium to encourage cell division and growth., Thus the cells in culture will produce an unorganised proliferative mass of cells, which is known as callus tissue., The cells that comprise the callus mass are totipotent, It may be able to regenerated back to normal plant by certain manipulations of, the medium and the cultural environment, Totipotency of a cell is the process of differentiation ie dedifferentiation and, redifferentation, Plasicity: It is the ability of the plant to alter their metabolism, growth and development to, best suit/adapt for the environment., , BASIC REQUIREMENTS, 1. Equipment and apparatus, , Culture vessel and glasswares, scissors, gas micro burner, autoclave, pH, meter, shaker, Balance, centrifuge, Incubator, Laminar air flow unit etc.
Page 2 :
2. Washing and storage facility, , Provision for fresh water supply and disposal of waste water and for storage, of glass wares- Separate cupboards or cabin, 3. Media preparation room, , Culture vessel, equipment, oven, autocalve, refrigerator, freezer for storage, of media and stock solution etc., 4. Sterilisation room, , Autoclave, Hot air oven, 5. Aseptic chamber (Inoculation room), , Laminar air flow cabinet having vertical or horizontal air flow – For aseptic, transfer with HEPA filter ( 0.3 µm ), 6. Incubation room, , B.O.D Incubator, , CULTURE MEDIA, For growth and development, Nutrient medium for plant cell is a well-defined mixture of inorganic salts,, organic salts, complex extracts, water, agar, plant growth regulators etc., Components of culture media, 1. Inorganic nutrients, , 15 elements important, Macro- Elements require in concentration greater than 0.5 mmol/l, Micro- Elements require in concentration less than 0.5 mmol/l
Page 3 :
Macronutrients, , 6 major inorganic nutrients are- N, P, K, Ca, Mg, S as salts, N concentration ------- 2-20mmol/l, Ca, P, S, Mg concentration----- 1-3 mmol/l, Micronutients, , B, Cu, Fe, Mn, Zn, Mo - Amount of Iron is more critical., Certain media requires Co, I and Na., One ion may be contributed by more than one salt (KNO3 and NH4NO3), Deficiency of inorganic salts can leads to certain symptoms in, callus:, culture NPK- Cell hypertrophy, Iron- Cessation of cell division, Boron- Retardation of cell division and cell elongation, 2. Organic Nutrients, a) Sugar, , Most commonly used carbon source is Sucrose at a Concentration of 2-5%, Glucose and fructose –Also used, Other carbon sources are Galactose, maltose, lactose, mannose, sorbitol, b) Vitamins and Aminoacids, , Added in trace amount., Essential vitamins- Thiamine(Vit B1), Pyridoxine (Vit B6),, Nicotinic acid( Vit B3), Calcium pantothenate ( Vit B5) and, ionositol., Amino acids- Arginine, Aspartic acid, Glutamic acid, glutamine or, methionine
Page 4 :
As they are heat sensitive - sterilized by Filtration sterilisation, 3) Complex extracts, , Coconut milk, Casein hydrolysate, Corn milk, Malt extract, Tomato juice, and Yeast extract, 4) Plant growth regulators, , Auxin- Cell growth and cell division, Cytokininins- Zeatin and Kinetin – Cell division, Gibberelins- Cell elongation, 4) Solidifying agents, Commonly - Agar as gelling agent for solidification of media., Agar conc. 0.8-1%, Others - Alginate, Carrageenan, Gelatin, Starch, Silica gel etc., Agar- Resistant to Enzymatic hydrolysis at incubation temp, also, neutral to media constituents., 5) PH, , Generally adjusted between 5 and 6 before sterilisation, More than 6 --------------- Hard medium, Less than 5-------------- do not allow satisfactory gelling of agar, , 6) Water, , Demineralised and double distilled water, Murashige and Skoog medium (MS Medium), Macronutrients, , Micronutrients, , Vitamins and aminoacids
Page 5 :
Ammonium nitrate 1650, , Boric acid, , Calcium chloride, 332.200, , Cobalt chloride, , Magnesium Sulphate, 180.690, , Copper sulphate 0.025, , Pottasium nitrate, , 1900 EDTA, , 6.200, 0.025, , 37.300, , Potassium phosphate 170 Ferrous sulphate 27.800, Manganes, 16.900, , Myo-Ionositol 100.00, Nicotinic acid 0.500, Pyridoxine, HCl, , 0.500, , Thiamine Hydrochloride 0.100, Glycine (Amino acid) 2.0, , sulphate, , Molybdic acid, , 0.213, , Potassium, iodide, , 0.830, , Zinc sulphate, , 8.600, , MEDIA PREPARATION, For stock solution – Each component weighed and dissolved separately, in water and then mixed together. All solutions stored in refrigerator till, used. PH and volume adjusted., For semisolid media – agar and sucrose are weighed and dissolved in, water by heating on water bath. Then other components stock solutions, added and PH adjusted., Sterilisation of culture media, Culture media packed in glass containers., Sealed with cotton plug And covered with Aluminium foil., Autoclaved at 2-2.2 atm pressure at 1210 C for 15-40 min., Exposure time- Varies depending upon volume of liquid
Page 6 :
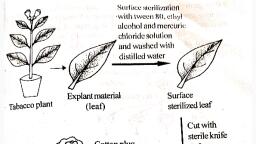
1) Preparation and sterilisation of explant, , Explant can be defined as a portion of plant which has been taken from the, plant to establish a culture., Explant grown in controlled environmental condition., Young shoots are more suitable as explants., Explant removed with sharp knifes, In case of seeds and grains they are directly sterilised and put in nutrient, medium., Surface sterilisation of explant, Ethanol (70%) - powerful sterilising agent but toxic, hence exposed for few, seconds or minutes, Sodium hypochlorite (0.5-1%)- Most frequently used, Mercury chloride (0.1%)- phytotoxic, rinse several, Hydrogen peroxide (30%) - strong sterilizing agent, Chromic acid, Calcium hypochlorite (3.25%- for 15 to 3o min)
Page 7 :
Usually Immersed in sterilising agent for 10 sec to 15 min. and washed with, distilled water. Repeat with sodium hypochlorite for 20 minutes , wash with, sterile water., 2) Sterilisation of glass ware tools/ vessels, , Glasswares- Dipped overnight in sodium dichromate- Sulphuric acidRemove dirt, wax or bacteria. Then Washed in running tap water, followed by distilled water and placed in inverted position in trays., Dried – Hot air oven – 1200 C for one hour., Plastic labwares- Mild abrasive detergent, followed by tapwater, rinsed, with alcohol, acetone etc., , 3) Culture media preparation and sterilization, , Culture media is prepared according to the formula and sterilized by autoclaving., 4) Production of callus from explant, , Sterilised explant transferred aseptically to defined medium., Incubated at 25 ± 20 C to maintain culture., Some amount of light is also necessary., Callus produced in 3-8 days of incubation, 4) Proliferation of callus, , Callus cut into small pieces and transferred to another fresh medium containing, an altered composition of hormone, which supports growth., 5) Subculturing of callus, , Periodically transferred to fresh medium for maintaining cell viability., Subculturing done at 4-6 weeks interval, 6) Suspension culture
Page 8 :
Contains uniform suspension of separate cells in liquid medium. For this, callus transferred to liquid medium, agitated continuously to separate cell., Types of plant tissue culture techniques, 1. Organ culture, 2. Protoplast culture, 3. Hairy root culture, 4. Callus culture, 5. Cell suspension culture, , ORGAN CULTURE, Isolated plant organs are grown under aseptic environmental conditions., Depending upon the plant organ, they are classified as, a) Root tip cultures, , , , , , , , , Tips of lateral roots sterilised and excised, Transferred to fresh medium., Lateral root continue to grow and provide several roots., After 7 days they are used to initiate stock culture or experiment., Thus the root material derived from a single radical., This type of genetically uniform cultured roots- clone of isolated roots
Page 9 :
a) Leaves or Leaf primordial culture, , Leaves detached from shoots (Young leaves have more growth potential)., Surface sterilised . Placed on solidified medium., b) Shoot tip culture, , Excised shoot tips are cultured on simple nutrient media containing, growth hormones., Further it develops into small shoots and these shoots can be rooted to, produce small plants or plantlets., Form roots and develop in to whole plants., c) Complete flower culture, , 2 days after pollination flowers excised., They are Sterilized using 5% calcium hypochlorite solution., Washed and transferred to agar medium., Fruits produced, but smaller than natural., Size increased by providing growth hormones to medium., d) Anther and pollen culture, , Young flower buds are removed from the plant and surface sterilized., Anthers carefully excised and transferred to nutrient medium., Very young and late stage of development anthers is generally, ineffective., Hence, for better response always select mature anther or pollen., Mature anther or pollen grains (microspora) of several species of, gymnosperms can be induced to form callus by spreading them out on, the surface of a suitable agar media.
Page 10 :
Mature anthers from angiosperms do not induce callus., e) Ovule and embryo culture, , Embryo is dissected from the ovule and put into culture media., Mature embryos require a simple nutrient medium containing mineral salts,, sugar and agar for growth and development., Very small embryos require hormones., The seeds are treated with 70% alcohol for about 2 min., Washed with sterile distilled water. Treated with surface sterilizing agent,, rinsed with sterilized distilled water., Kept for germination by placing them on double layers of pre sterilized, filter paper placed in petridish moistened with sterilized distilled water or, placed on moistened cotton swab in petridish., The seeds are germinated in dark at 25–28°C and small part of the seedling, is utilized for the initiation of callus., f) Meristem culture, , Culture of extreme tip of the shoot,is used as technique to free plants free from, virus infections., Explants are dissected from either apical or lateral buds., They comprise a very small apex (0.2-1.0 mm) consisting of just apical, meristem and one or two leaf primordia., Further it develops into small shoots and these shoots can be rooted to produce, small plants or plantlets., PROTOPLAST CULTURE, Protoplasts are naked plant cells without the cell wall, but they possess plasma, membrane and all other cellular components., Sources of protoplasts: Leaves, roots, shoot apices, fruits, embryos and, microspores.
Page 11 :
The technique involves the following stages, 1. A small piece of epidermis from a plant is selected., 2. Isolation of protoplast, , Isolation of Protoplasts: Protoplasts are isolated by two techniques, 1. Mechanical method, 2. Enzymatic method, , Mechanical Method:, Tissue immersed in 1 M sucrose solution untill protoplasm shrink away from, cell wall., Plasmolysed tissue cut with sharp knife. Undamaged protoplast are released by, osmotic swelling., This method is no more in use because of the following limitations:, Yield of protoplasts and their viability is low., It is restricted to certain tissues with vacuolated cells., , Enzymatic method, 1. Two step or sequential method:, Cells are first isolated from callus or tissue by using pectinase ., To this cell suspension cellulase is added to digest the cell wall and release, protoplasts., 2. One step or simultaneous method:, By using two enzymes, cellulase and pectinase simultaneously.
Page 12 :
Scheme of somatic cell hybridization, Protoplasts of different species can be fused to generate a hybrid and this, process is referred to as somatic hybridization (or protoplast fusion)
Page 13 :
Application, 1. DNA transformation, 2. Used to study wall synthesis and decomposition, 3. Ingesting foreign material into cytoplasm, 4. Used to study incompatable species, 5. Ingesting foreign materials into cytoplasm, 6. Culture of nematodes, , HAIRY ROOT CULTURE, It is the culture produced after the infection of explant or culture by the gram negative, soil bacterium Agrobacterium rhizogenes., Agrobacterium rhizogenes is causative agent of hairy root syndrome., It is a common soil organism capable of entering of plant through a wound and, causing a proliferation of secondary roots., Steps, Explants are wounded and then inocculated with Agrobacterium rhizogenes, Usually 2 or 3 days later explant transferred into solid media with antibiotics,, such as cefotaxime, vancomycin or ampicillin to kill or eliminate reductant, bacteria., The hairy roots will be induced within a short period of time,which varies from, one week to over a month depending on different plant species, The decontaminated hairy roots can be subcultured on phytohormone free, medium
Page 14 :
Genetics of hairy root culture, Agrobacterium recognizes some signal molecules exuded by wounded plant cells, and becomes attached to it., The bacteria contain the root inducing plasmid ( Ri- plasmid)., The bacteria genetically transfer part of Ri-plasmid called the transfer DNA ( TDNA) to the plant genome, where it expressed and make the plant cell to Proliferate, by increasing the rate of cell division( cytokine expression) and cell elongation (, auxin expression) to produce hairy roots., They Produce the opines which is a type of unusual amino acids( octopine, agropine,, nopaline, mannopine, and cucumopine) which is used by the bacterium as a carbon,, nitrogen and energy source., Bioreactors of hairy root culture :Air lift bioreactor, Stirred tank bioreactor, Bubble column, , Applications, Functional analysis of genes, Expressing foreign proteins, Production of secondary metabolites eg: shikonin production by hairy root culture of, lithospermum erythrorhion (used as antibacterial and antiulcer agents), Production of ginsenosides, beriberine, rosmarinic acid, quinine, cardenolides, Can be used to regenerate whole plant, CALLUS CULTURE, The cut surface or wounded plant produces a proliferation of undifferentiated cell at the, wounded site. These masses of cells are known as callus. It is an amorphous, aggregate of loosely arranged parenchyma cells, which proliferate from mother cells., Callus can be subdivided into two categories: compact and friable., In compact callus, cells are densely aggregrated, while in friable callus, the, connection is more loose., Friable callus is soft and breaks apart easily.
Page 15 :
Steps involved, a) Selection and preparation of explant, , Selection: Organ or culture is selected such as segments of root or stem, leaf, primordia, flower structure or fruit, etc., Preparation of explant, Excised parts of the plant organ are first washed with tap water., Then sterilized with 0.1% of mercuric chloride (HgCl2) or 2% w/v,, sodium hypochlorite solution for 15 min., If waxy layer present- pretreatment with 70 % alcohol or tween 20, (wetting agents)., Wash the sterilized explants with sterile distilled water and cut, aseptically into small segments (2–5 mm)., b) Selection of culture media, , , , , , , , , , MS medium:, High concentration of nitrate, potassium and ammonium ions are preferred in MS, medium in comparison to other media., Growth hormones : Auxins (IBA and NAA) for rooting ., Growth regulators such as auxins(2, 4-D and 2, 4, 5-T, NAA,IBA ) and, Cytokinins are added., Auxins+cytokinins - shoot proliferation., The pH of the medium is adjusted (5.0–6.0) and poured into culture vessels., Vessel is cotton Plugged and sterilized by autoclaving., , c) Transfer of explant ( Inocculation), , Surface sterilized organs (explant transferred aseptically into the vessel containing, semisolid culture medium, d) Incubation, , The inoculated vessels are transferred into BOD incubator at 25–28°C using, light and dark cycles for 12-h duration .
Page 16 :
After three to four weeks, callus should be about five times the size of the, explant., The unique feature of callus is its ability to develop normal root and shoot,, ultimately forming a plant., Callus formation occurs by three stages, Induction- cell matabolic activity increase, Cell division and –explant changes into meristematic state, Cell differentiation- morphological and physiological, differentiation-Thus secondary metabolites production, Applications, Production of plantlets, For obtaining virus-free plants, For generation of useful somoclonal and gametoclonal varients, , , , , , , As source of protoplast and suspension culture, Production of useful secondary metabolites, For biotrasformation studies, For conservation of germplasm, For mutagenetic studies, SUSPENSION CULTURE, , Suspension culture is a type of culture in which single cells or small aggregates of, cells multiply while suspended in agitated liquid medium., Suspension cultures are established from friable callus in liquid medium., Liquid cultures must be constantly agitated, generally by a gyratory shaker, at 100-250 rpm (revolution per minute).to facilitate aeration and dissociation, of cell lumps into smaller pieces.
Page 17 :
Need to be subcultured about every week, allow more accurate determination, of the nutritional requirements of cells., , Suspension culture is termed to be good suspension if it contains a high percentage of, single cells and is cultivated in optimum environmental conditions, , GROWTH PHASE OF CULTURE, Plant cell suspension and callus cultures show an ideal growth curve or growth, cycle comprising different growth phases., Lag phase, Log phase/exponential phase
Page 18 :
Linear phase, Stationary phase, Decline phase, , Lag phase, It is characterised by acclimatization of explants in the nutrient medium, under invitro conditions., In case of callus culture, it can be exhibited by swelling or, enhancement of explants dimention whereas, in suspension cultures, callus gets familiar to culture conditions, Log phase/exponential phase, In this phase there is a rapid increase in number and cell biomass., Linear phase, Linear phase exhibit maximum growth and the linear pattern with respect, to time is observed., Nutritional uptake is maximum at this stage
Page 19 :
Stationary phase, This phase represents a stage where rate of cell division or biomass, production is equal to the rate of cell death., In some cases secondary metabolites accumulation is maximum in late, stationary phase., Decline phase, This phase represents more cell death than cell division and cell, reproduction., Secondary metabolite metabolites accumulation is maximum in early, decline phase., Application, For study of cell behavior ( including cytology, nutrition, , ,metabolism, morphogenesis, embryogenesis and pathology), Production of useful secondary metabolites, For applying various biotechnological strategies like precursor feeding,, , elicitation, immobilization, biotrasformation studies for enhanced production of, secondary metabolites., Selection of cell lines with valuable properties such as resistance to disease,, , herbicides, overproduction of secondary metabolites.
Page 20 :
Applications of plant tissue culture