Page 1 :
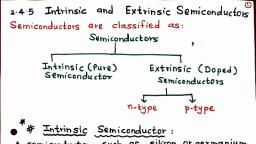
854, MOVE FAST WITH PHYSICS-XIl, 14.1 Elemental and Compound Semiconductors, H, Bands of Metals, Insulators and Semiconduc, Mechanism of Conduction, Doping, Extrinsic, and p-type Semiconductors and their Energy, 14.1.1 Electronic devices Any device whose action is based, on the controlled flow of electrons through it is called an, electronic device. The branch of physics that deals with the, study of these electronic devices is called electronics. The, electronic devices are of two types:, (i) Vacuum tubes. These include vacuum diode, triode,, tetrode, etc. In a vacuum tube, electrons obtained froma, heated cathode are controlled by varying voltages between, its different electrodes. These devices are bulky, consume, high power, operate generally at high voltages, have, limited life and low reliability., (ii) Solid-state electronic devices. In such devices, the, charge carriers flow through solid-state semiconductors. These, devices include junction diodes, transistors and integrated, circuits. These are small_in_size, consume low power,, operate at low voltages, have long life and high reliability., 14.1.2 Classification of solids on the basis of their, resistivity values :, A. Metals. They have very low resistivity or high, conductivity., p=10-2-10-8 2m; o=102 -10° Sm, B. Insulators. They have high resistivity or low, conductivity., p=10° 2m; ox10-8 Sm', C. Semiconductors. They possess resistivity or conduc-, tivity intermediate to metals and insulators., p=105 -10° Qm; ox10-5 -10° Sm-1, -1, Some distinguishing features of the semiconductors:, (iy Semiconductors have a much higher resistivity, than metals., (iiy Semiconductors have a temperature coefficient of, resistivity (a) that is both negative and high. That is, the resistivity of semiconductors decreases rapidly, with temperature., (iiy Semiconductors have, number density n of charge carriers than metals., a, considerable lower, 14.1.3 Classification of semiconductors on the basis of, their chemical composition :, A. Elemental semiconductors : Si and Ge., B. Compound semiconductors : Examples are.:, (i) Inorganic : CdS, GaAs, CdSe, InP, etc., (ii) Organic polymers : Polypyrrole, polyaniline, poly-, thiophene, etc., Scanned by CamScanner
Page 2 :
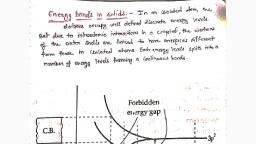
855, bands are separated by regions in (iő Semiconductors, The empty conduction band, CHAPTER 14: SEMICONDUCTOR ELECTRONICS, energy, The allowed, separated from the filled valence band by a small, energy gap (E, <3eV) Some electrons of the, valence band easily get thermally excited to the, conduction band and can conduct electricity. So, semiconductors acquire small conductivity even at, room temperature., hand,, For Si, E =1.17 eV and for Ge, E, =0.74 eV., zero is called Fermi level and the energy corresponding to the, Here E, =0., Fermi level is called Fermi Energy. For intrinsic semicon-, This makes available a large number of free ductors, Fermi level lies in the middle of the forbidden, high conductivity or low resistivity., Insulators. Here the conduction band is empty and ductors in which the electrical conductivity is totally governed, the valence band is filled. The forbidden energy by the thermally excited electrons and consequently created holes, is large (E, >3 eV). Electrons cannot be and in which no impurity atoms are added to increase their, 14.1.7 (a) Intrinsic semiconductors, The pure semicon-, gap, excited from the valence band to the conduction conductivity are called intrinsic semiconductors and their, band even by applying a strong electric field. conductivity is called intrinsic conductivity., Therefore, no electrical conduction is possible., (b) Valence bond model of intrinsic semiconductors In, a crystal of germanium, each Ge atom is tetrahedrally, bonded to four neighbouring Ge atoms, as shown in, Fig. 14.3. Such a structure with all bonds intact exists at, low temperature., For diamond, E, =6 eV., Partially filled, conduction band, Filled valence, band, (i), + 4, +4, Overlapping, conduction band, Filled valence, band, +4, + 4, (ii), (a) Metals, +4, + 4, + 4, Empty, conduction, band, E. <3 eV, Filled, valence, band, Fig. 14.3 Covalent bonding in Si or Ge., The symbol + 4 represents inner core of Ge or Si., All bonds are intact at low temperature., (b) Insulators, Empty, conduction, band, As the temperature increases, the thermal energy of the, valence electrons increases. As shown in Fig. 14.4, an, electron may break away from the covalent bond and, becomes free to conduct electricity. This electron leaves, behind a vacancy in the covalent bond (at site 1). This, vacancy of an electron with an effective positive electronic, charge is called a hole, It behaves as an apparent free particle, with a charge +e., E, >3 eV, Filled, valence, band, (c) Semiconductors, Fig. 14.2 Energy band diagrams., Scanned by CamScanner
Page 3 :
856, MOVE FAST WITH PHYSICS-XII, sá) Energy band diagram of intrinsic semiconducto,, At T=0 K, the valence band of a semiconductor i, completely filled with electrons while the conduction band, is empty, as shown in Fig. 14.6(a). Hence an intrinsie, semiconductor behaves like an insulator at T=0 K A, higher temperatures (T>0 K), some electrons of the, valence band gain sufficient thermal energy and jump to, the conduction band, creating an equal number of holes in, the valence band. These thermally excited electrons, Thermally generated, free electron, +4, Hole at, site 1, (electron, vacancy), + 4) Site 1, Site 2, E, E, Fig. 14.4 Generation of a hole at site 1 and liberation of a free, electron due to thermal energy at moderate temperature., E,, E,, As each free electron creates one hole, so in an intrinsic, semiconductor, the number density of free electrons (n,) is, equal to the number density of holes (n, ) and each is equal, to the intrinsic charge carrier concentration (n;)., (b) at T>0K, (a) at T=0K, (behaves like insulator), n. =n, = 1;, of, Fig/14.6 Generation of a hole at site 1 and liberation of a free, electron due to thermal energy at moderate temperature., Mechanism, conduction, in, intrinsic, (c), semiconductors, Electrical conduction in an intrinsic occupy the lowest possible energy levels in the conduction, semiconductor is due to electron-hole pairs. Fig. 14.4 band. Therefore, the energy band diagram of an intrinsic, shows a hole at site 1. An electron at site 2 of the semiconductor at T>0 Kis of the type shown in Fig. 14.6(b)., neighbouring covalent bond may jump to the site 1. After Clearly, the number of electrons in conduction band is, such a jump, a hole is created at site 2 and site 1 gets equal to the number of holes in valence band., occupied by an electron as shown in Fig. 14.5. Apparently,, the hole has moved from site 1 to site 2. Under the action of developing semiconductor devices :, an applied electric field, the holes move in the direction of, the electric field (due to jumping of bound electrons in the, reverse direction from one atom to another). So they act as, positive charge carriers and give rise to a hole current I,., The thermally generated free electrons give rise to an, independent electron current I,. The total current is, (e) Limitations, of intrinsic semiconductors in, (i) Intrinsic semiconductors have low intrinsic charge, carrier concentration (of hole and electrons), =106 m-3. So they, have, low, electrical, conductivity., (ii)/As intrinsic charge carriers are always thermally, generated, so flexibility is not available to control, their number., I= Electron current + hole current = I, + I,, %3D, (ijiy For intrinsic semiconductors, n, =n,. They cannot, have predominant hole or electron conduction., This puts a limit to the usefulness of such, +4, +4, materials., 14.1.8 Doping The process of deliberate addition of a desirable, impurity to a pure semiconductor so as to increase its, conductivity is called doping. The impurity atoms added are, called dopants and the semiconductors doped with the impurity, atoms are called extrinsic or doped semiconductors., Site 1 •, + 4, +4, Site 2-, ....., +4, + 4, Essential requirements for a doping process:, (Y The semiconductor material should be of very, high purity, 99.9999% or more., GY The dopant atom should neatly replace the, semiconductor atom., ....., Fig. 14.5 Apparent movement of a, hole in an intrinsic semiconductor., Scanned by CamScanner, Electron energy
Page 4 :
the tetravalent Si atom, it uses four of its five valence, Hole, pure sample of a molten semiconductor., The size of the dopant atom should be almost the electrons in forming four, ( The dopant atoms should not distort the crystal required to detach this electron. At room temperature, the, same as that of the semiconductor atom. For this neighbouring Si atoms while the fifth electron is loosely, the atoms of third and fifth group of the periodic bound to the impurity atom. A very small amount of, 857, CHAPTER 14 : SEMICONDUCTOR ELECTRONICS, bonds, with, covalent, table are most suitable., ionisation energy (001 eV for Ge and 0. 05 eV for Si) is, thermal, energy is enough to set free this electron. The, lattice., each pentavalent impurity atom donates one extra electron, for conduction, it is called a donor. These semiconductors, have free electrons contributed by donors and generated, YBy adding the impurity atoms to an extremely by the thermal process while the holes are only due to, thermal generation. Hence, the electrons are the majority, charge carriers and holes are the minority charge carriers. As, most of the current is carried by the negatively charged, small, about 1 part per million., Methods of doping :, () By heating the crystalline semiconductor in an, aimosphere containing dopant atoms or their electrons, so the semiconductors doped with donor type, molecules so that the dopant atoms diffuse into impurities are known as n-type semiconductors., the semiconductor., For n-type such semiconductors, n. >> n,., (iiY By bombarding the semiconductor with the ions of, dopant atoms, the dopant atoms can be implanted, into the semiconductor., B. Formation of p-type semiconductor. Such, semiconductor is obtained by doping the tetravalent, semiconductor Si (or Ge) with trivalent impurities such as, In, B, Al or Ga. As shown in Fig. 14.8, the impurity atom, uses its three valence electrons in forming covalent bonds, with three neighbouring Si atoms and one covalent bond, with a neighbouring Si atom is left incomplete due to the, deficiency of one electron. An electron from the neigh-, bouring Si-Si covalent bond can slide into this vacant bond,, 14.1.9 Extrinsic semiconductors A semiconductor obtained creating a vacancy or hole in that bond. This hole is now, by doping a pure semiconductor with acceptor or donor impurity available for conduction. The trivalent impurity atom, atoms so as to increase its conductivity is called an extrinsic becomes negatively charged when all its valence bonds get, filled. The trivalent impurity atom is called an acceptor because, a, Two types of dopants :, O Pentavalent dopants such as As, Sb and P. These are, also called donors., (iY Trivalent dopants such as In, B and Al. These are, also called acceptors., semiconductor. Extrinsic semiconductors are of two types :, Y-type semiconductors. These are the semiconductors it creates a hole which can accept an electron from the, obtained by doping Ge or Si with pentavalent dopants. neighbouring bond. Obviously, there are holes created by, Yptype semiconductors. These are the semiconductors, obtained by doping Ge or Si with trivalent dopants., the acceptor atoms in addition to the thermally generated, holes while the free electrons are only due to thermal, generation. Hence, holes are the majority charge carriers and, This electrons are the minority charge carriers. The semicon-, semiconductor is obtained by doping the tetravalent ductors doped with acceptor type impurities are called, raconductor Si (or Ge) with pentavalent impurities such p-type semiconductors, because most of the current in these, AS, Por Sb of group V of the periodic table. As shown in semiconductors is carried by holes which have effective, A. Formation, of n-type semiconductor., 147, when a pentavalent impurity atom substitutes positive charge., For p-type semiconductors, n, >>1.., + 4, + 4, + 4, Unbonded 'free', electron, donated by, pentavalent, (+ 5 valency), + 4, + 4, +5, +4, Electron., atom, +4, +4, +4, + 4, Fig. 14.7 Formation of n-tvpe semiconductor by, doping tetravelent Si with pentavalent impurity., Fig. 14.8 Formation of p-type semiconductor by, doping tetravelent Si with trivalent impurity., Scanned by CamScanner
Page 5 :
858, MOVE FAST WITH PHYSICS-XII, 14.1.10 Thermodynamic relation between the number, densities of electrons and holes for an extrinsic, semiconductor When conduction electrons and holes are, created in a semiconductor, a process of destruction occurs, simultaneously in which electrons and holes recombine, with each other. At equilibrium, the rate of generation of, charge carriers is equal to the rate of destruction of charge, E., Ep, 0.01 eV, = 0.01 - 0.05 eV, EA, E,, E,, carriers., For an extrinsic semiconductor,, (a), (b), Rate of recombination cn,n,, Fig. 14.9 (a) Energy band diagram of n-type semiconductor at T >0K, (b) Energy band diagram of p-type semiconductor at T >0 K., or,, rate of recombination =, .(1), covalent bond i.e., a very small energy (×0.01-0.05 eV) is, required by an electron of the valence band to move into, For an intrinsic semiconductor, n =n, =n;, so the this hole. Hence the acceptor energy level E, lies slightly, above the top of the valence band, as shown in Fig. 14.9(b)., At room temperature, many electrons of the valence band, get excited to these acceptor energy levels, leaving behind, As long as the lattice structure of the semiconductor equal number of holes in the valence band. These holes can, remains the same, the rates of recombination given by conduct current. Thus the valence band has more holes, where Ris a constant known as recombination coefficient., equation (1) becomes, Rate of recombination =, Rn?, ...(2), equations (1) and (2) for extrinsic and intrinsic than electrons in the conduction band., semiconductors must be equal., Holes The vacancy or absence of an electron in the, ..(3) bond of a covalently bonded crystal is called a hole. In terms of, The above equation implies the following facts about band theory, whenever an electron is removed from the, completely filled valence band of a semiconductor, a, 14.1.12, Hence Rn n, = Rn,, or, n,11, =n?, -type and p-type semiconductors :, (1) For an n-type semiconductor, n, is necessarily vacancy is left behind in the valence band. This vacancy, serves as a positive charge carrier and is called a hole., yet its product with n, remains, equal to n. This is possible only if n, becomes less, This implies that the number of holes gets, greater than, and, Characteristics of holes :, than, YA hole is just a vacancy created by the removal of, an electron from a covalent bond of semiconductor., suppressed in n-type semiconductors., (ii) For a p-type semiconductor, n, is necessarily, greater than n; and yet its product with, equal to n. This is possible only if n, becomes less, (iiIt has the same mass as the (removed) electron., (iii) It is associated with a positive charge of, magnitude e., ne, remains, than, Thus the number of electrons is, (iyy The energy of a hole is higher, the farther below it, is from the top of the valence band., suppressed in a p-type semiconductor., 14.1.11 Energy band diagram of n-type semiconductor, In n-type semiconductors, the extra (fifth) electron is very with temperature The conductivity of a semiconductor is, weakly attracted by the donor impurity. A very small given by, energy (2001 eV) is required to free this electron from the, donor impurity. When freed, this electron will occupy, the lowest possible energy level in the conduction band i.e.,, the energy of the donor electron is slightly less than E of electrons and holes decrease due to the increase in the, Thus the donor.energy level E lies just below the bottom of the, conduction band, as shown in Fig. 14.9(a). At room, temperature this small energy gap is easily covered by the semiconductors, more and more electrons [nce"8™], thermally excited electrons. The conduction band has more, electrons (than holes in valence band) as they have been The increase in carrier concentrations,, contributed both by thermal excitation and donor impurities., 14.1.13, Variation of conductivity of a semiconductor, =e(n. H. + n, H6), As the temperature increases, the mobilities u, and H, collision frequency. But due to the small energy gap, of, from the valence band cross over to the conduction band., is so large, that the decrease in the values of u, and u, has no, Energy band diagram of p-type semiconductor In influence. The overall effect is that the conductivity, p-type semiconductors, each acceptor impurity creates a increases or the resistivity decreases with the increase or, and, hole which can be easily filled by an electron of Si-Si temperature., Scanned by CamScanner, Electron energy