Page 1 :
ENGINEERING CHEMISTRY, Module IV Unit II, Alloys, Alloys – purposes of alloying, application of alloys like Brass, Bronze and Solder.
Page 2 :
What is an alloy?, An alloy is a homogeneous mixture in which at least one component is a metal., ALLOYS
Page 3 :
Purpose of making alloys, ALLOYS, 1. Enhance the hardness of a metal: An alloy is harder than its components., , , 2. Lower the melting point: Pure metals have a high melting point. The melting point lowers when pure metals are alloyed with other metals or nonmetals., , , , 3. Enhance tensile strength: Alloy formation increases the tensile strength of the parent metal.
Page 4 :
Purpose of making alloys, ALLOYS, 4. Enhance corrosion resistance: Alloys are more resistant to corrosion than pure metals., , , 5. Modify color: The color of pure metal can be modified by alloying it with other metals or nonmetals containing suitable color pigments., , , 6. Provide better castability:Metals need to be alloyed to obtain good castings because , alloys expand on solidification.
Page 5 :
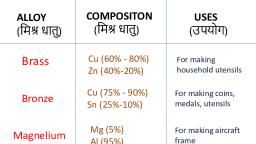
Composition of alloys like Brass, Bronze, Duralumin and Solder, Brass – Mainly copper with up to 50% Zinc. Used for making inexpensive jewellery, hose nozzles, couplings and stamping dyes., ALLOYS
Page 6 :
Composition of alloys like Brass, Bronze, Duralumin and Solder, Bronze – Mainly copper with up to 12% Tin. Used for making coins, medals, heavy gears, tools and electrical hardware., ALLOYS
Page 7 :
Composition of alloys like Brass, Bronze, Duralumin and Solder, Solder – 63% Tin ,37% Lead. , Due to low melting point it can be used for joining two metal junctions at low temperature. Solder is widely used in assembling the electronic components in to circuit boards in electronic industry. Since the temperature is low, sensitive components are not damaged in the soldering process., ALLOYS
Page 8 :
Review questions, , What are alloys?, Discuss the main purposes of alloying., 3. Give the composition and application of alloys , i) Brass, ii) Bronze, iii) Solder, ALLOYS