Page 1 :
Advances in Phytochemistry First Semester 2020/2021 Session, , A. Ahmed, , Phytochemistry is an interface (discipline) between chemistry and botany which deals with chemical, substances produced by plants. Phytochemicals are chemical compounds in plants. They are also, known as plant metabolites, natural products or plant constituents., Why is phytochemistry important?, Phytochemicals are known to provide to the plants’ protection against insects’ attack and plant, diseases. they serve as lead for development of new drugs, herbicides, preservatives, toxins, dyes,, tanning materials, biofuels etc. Some phytochemicals are toxic to man and animals. They serve as, markers for taxonomic studies (chemotaxonomy)., Why phytochemicals are classified based on their pharmacology, On the basis of their pharmacological properties, plants can be classified as antimalarials (e.g., cinchona), antineoplastics (e.g. catharantus), cardiovascular etc. The pharmacological properties of, plants are linked to their constituents. Examples include quinine from cinchona, artemisinin from, Artemisia annua (antimalarial), Taxol from Taxus brevifolia (antitumor), berberine from Berberine vulgaris, (antidiabetic), piperidine from Piper nigrum (antiepileptic) etc. Phytochemicals are sometimes isolated, in pure form for drug development. In the early years of the 19thcentury, the first compound isolated, was morphine from opium in 1803., Antimalarial Drugs, Malaria is a life-threatening parasitic disease transmitted by mosquitoes. There are four types of, human malaria parasites — Plasmodium vivax, P. falciparum, P. malariae, and P. ovale of which the first, two are the most common, and P. falciparum the deadliest type of malaria infection., Most of the currently used antimalarial drugs have been developed from knowledge and, investigation of medicinal plants. Malaria was among the first diseases to be treated by a pure, chemical compound—quinine—isolated from the cinchona bark in 1820., Cinchona, Quinine was first isolated by the French scientists Pelletier and Caventou from the bark of the, Cinchona spp. (Rubiaceae) tree that was used by Peruvian Indians. There are about 23 species of, cinchona which include C. calisaya, C. officinalis, C. legdgeriana, C. succirubra. Other alkaloids contained, in cinchona bark include quinidine, cinchonine and cinchonidine.
Page 2 :
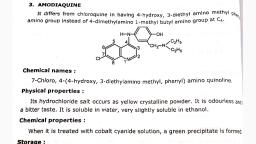
The structure of quinine was established by Rabe in 1908 and was used as a template for the, synthesis of Chloroquine (CQ) in 1940. Despite the prevalence of CQ-resistant P. falciparum strains,, it was, until recently, the only drug available for malaria chemotherapy., , Quinine, , Chloroquine, , Properties of Quinine and other Cinchona Alkaloids, Quinine is a cinchona alkaloid that belongs to the aryl amino alcohol group of drugs. It is very, slightly soluble in H2O, soluble in ethanol, chloroform, ether, benzene and other organic solvents. It, is an extremely basic compound and is, therefore, always presented as a salt., It forms 2 types of sulfates:, Quinine monosulfate (neutral and H2O insoluble)., Quinine bisulfate (acidic and H2O soluble)., Various preparations exist, including the hydrochloride, dihydrochloride, sulphate, bisulphate, and, gluconate salts; of these the dihydrochloride is the most widely used. Quinine has rapid, schizonticidal action against intra-erythrocytic malaria parasites. It is also gametocytocidal, for Plasmodium vivax and Plasmodium malariae, but not for Plasmodium falciparum. Quinine also has, analgesic, but not antipyretic properties. The anti-malarial mechanism of action of quinine is, unknown.
Page 3 :
Quinine and other cinchona alkaloids including quinidine, cinchonine and cinchonidine are all, effective against malaria. The efficacies of these four alkaloids were evaluated in one of the earliest, clinical trials, conducted from 1866 to 1868 in 3600 patients using prepared sulfates of the alkaloids., However, after 1890 quinine became the predominantly used alkaloid, mainly due to a change in, supply from South American to Javan cinchona bark, which contained a higher proportion of, quinine. Quinine remained the mainstay of malaria treatment until the 1920s, when more effective, synthetic antimalarials became available. The most important of these drugs was chloroquine, which, was extensively used, especially beginning in the 1940s. With heavy use, chloroquine resistance, developed slowly. Resistance of Plasmodium falciparum to chloroquine was seen in parts of Southeast, Asia and South America by the late 1950s, and was widespread in almost all areas with falciparum, malaria by the 1980s. With increasing resistance to chloroquine, quinine again played a key role,, particularly in the treatment of severe malaria. To-date quinine continues to play a significant role in, the management of malaria., Uses of Quinine and other Cinchona Alkaloids, Quinine is used mainly as anti-malarial in a dose of 2g of quinine sulfate or other salt for 14, days., Quinidine is used as a cardiac depressant (anti-arrhythmic), particularly to inhibit auricular, fibrillation in a dose of 0.6-1.6 g of quinidine sulfate daily., Cinchonine and cinchonidine are used as anti-inflammatory.
Page 4 :
Artemisia annua L. (Asteraceae), , Artemisia annua (qinghaosu) has had many traditional uses in China for several millennia. Artemisinin, was first isolated in pure form in 1972 and since then has been in use for treatment of chloroquineresistant malaria without any adverse side effects. Artemisinin represents a new structure of, antimalarial pharmacophore that comprises an endoperoxide sesquiterpene lactone., , , The functional group associated, with, the, activity, is, the, endoperoxide., , , , The effectiveness of naturally, occurring artemisinin is limited by, its insolubility and bioavailability., , The major metabolites of A. annua are artemisinin (1), artemisinic or arteanuic acid (2), arteannuin B, (3) and dihydroarteannuin (4). Of these four substances, only artemisinin (1) is biologically active, and has been converted into several other derivatives, which are more effective and are now in, clinical use. Cultivation of Artemisia annua is necessary because its chemical synthesis is quite, laborious and costly., , (1), , (2), , (3), , (4), , Other naturally occurring peroxides from other members of the Asteraceae (Artemisia sp., Achillea, millefolium, Anthemis nobilis, Heterothalamus psiadioides), have been tested but it was found that although, all of them show some activity, none was as active as artemisinin. Today both quinine (derived from
Page 5 :
the cinchona bark) and artemisinin (from qinghaosu) remain of prime importance in the control of, malaria.