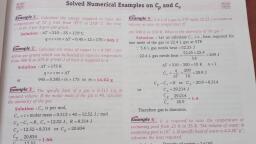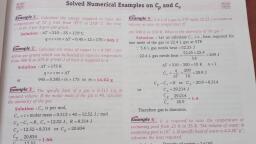Page 1 :
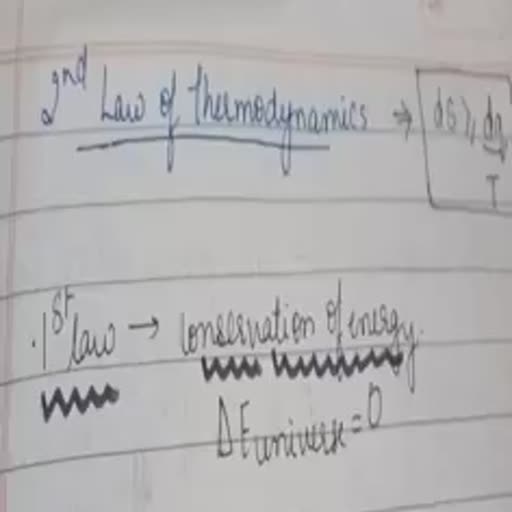
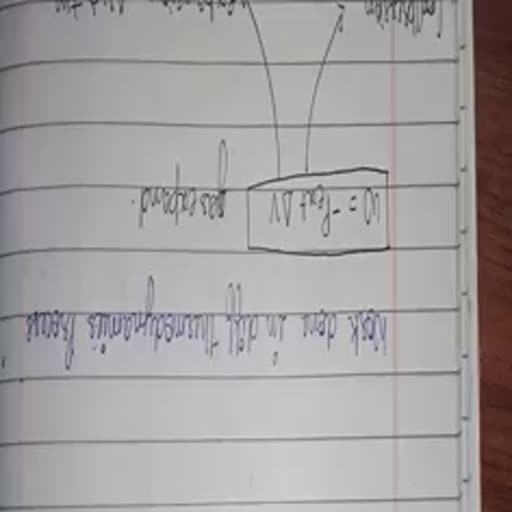
& 2+, , «#° Solved Numerical Examples on C, and Cy one, , Example 1_ Calculate the ene ; _, Re ic CHEF SY ed a to raise the Example 4 5 61 of a gas at STP needs 52.25 J at constant, , , , , : 2 gir Be se, Solution : AT =210 —35=175°C om 300 K to 310 K. What is the atomicity of the gas ?, q=cxmx AT =0.45x12x175=945 J Solution : Let us calculate C, i.e., heat required for, a one mole of the gas or 22.4 L gas at STP, Example 2 _ Calculate the mass of copper (c = 0.385 J per -: 5.6 L gas needs heat =52.25 J, kelvin per gram) which can be heated to raise its temperature .22.4 L gas needs heat = 5225 x 22.4 =209 J, 6, , from 300 K to 475 K if. 945 J of heat is supplied to it, AT =310-300=10K n=1, , , , , , , , Solution : AT =175 K, q=cxmxAT watts q _ 209 -209 J, or 945 =0.385xmx175 or m= 14.02 g AE 10., in C,—-C,=R or C, —20.9 =8314, Example 3 _ The specific heat of a gas is 0.313 J/g at or C, =29214 J, constant volume. If the molar mass of the gas is 40, calculate Cc, he atomicity of the gas Z Vp _ 29214 _ 1.4, Ee se = ee, Solution : C, is per mol, mews eee, e€ t :, C, =cx molar mass = 0.313 x 40 =1252 J/ mol ape a nena, a, Cp -C, =R, C, =1252 J, R=8314 J Example 5_ It is required to raise the temperature of, C. —12.52 =8314 or C, =20834 swimming pool from a2 K to 35 K. The volume of water mn, ; P v 5 —1, C, 20834 swimming pool is 10° L. If specific heat of water ts 4ulke. ae, = = _ = = 1.66. calculate the heat ee, z Solution ; Density of water =1 g/mL, , Therefore gas is monoatomic. Volume of water = 102=10° =10" ue