Page 1 :
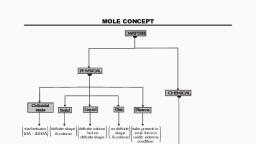
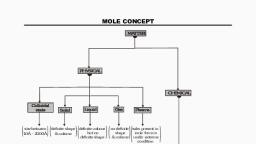
MOLE CONCEPT
Page 2 :
MOLE, , The mole is the amount of substance of a system which, contains as many elementary entities as there are atoms, in 12gm of carbon 12., , The quality of a substance in which 6.023×10²³ particles, of that substance are present, it is called a mole or a, mole quantity., 1 mole quantity = 6.023× 10²³ particles
Page 3 :
AVOGADRO NUMBER, The number of particles present in one mole of a substance, is called the Avogadro number., It is denoted by N.A and it’s numerical value is 6.023×10²³, , N.A = 6.023×10²³ = 1 mole quantity
Page 4 :
MOLAR VOLUME, The Volume occupied by 1 mole of any gas at N.T.P is called, molar Volume., The value of molar Volume = 22.4ltr = 22400 ml, , Molar mass : the mass occupied by I mole of any substance at, N.T.P is called molar mass.
Page 5 :
FORMULA TO GET MOLES, Number of moles (n) = w/m, Where ,, w= gram weight of substance, m = molecular or atomic weight, , Number of moles (n) = V/22.4, Where,, V = volume of gas in litre, , Number of moles (n), , = N / NA, , Where,, N = number of particles, N.A = Avogadro number
Page 6 :
Number of molecules in given moles = Mole × N.A, , Number of atoms in given mole = mole × N.A × number of atoms in a molecule, , Note Below
Page 13 :
Find the number of molecules in 4.4 GM CO2
Page 14 :
Find the number of moles and atoms (4.6gm Na)
Page 15 :
Which of the following contains maximum number, of atoms ?, (1) 4 g of H2, (2) 16 g of O2, (3) 28 g of N2, (4) 18 g of H2O
Page 16 :
10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded., Amount of water produced in this reaction will be :(1) 1 mol, , (2) 2 mol, , (3) 3 mol, , (4) 4 mol, , AIPMT 2009
Page 17 :
Which has the maximum number of molecules among the, following ?, (1) 64 g SO2, , (2) 44 g CO2, , (3) 48 g O3, , (4) 8 g H2, , The number of water molecules is maximum in :(1) 18 g of water, (2) 18 mol of water, (3) 18 molecules of water, (4) 1.8 g of water, , AIPMT Mains 2011, , NEET 2015
Page 18 :
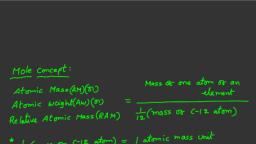
ATOMIC MASS, , It define as the number which indicates how many times the mass of one, atom of an element is heavier in comparison to 1/12 th part of the mass of, one atom of C12.
Page 19 :
ATOMIC MASS UNIT, The quantity of 1/12th mass of an atom of C12 is known as, atomic mass unit., , 1.67 × 10–24 g = 1 a.m.u.
Page 20 :
GRAM ATOMIC MASS, , When numerical value of atomic mass of an, element is expressed in gram then the value, becomes gram atomic mass.
Page 21 :
MOLECULAR MASS, , The number which indicates how many times the mass of, one molecule of a substance is heavier in comparison of, 1/12th part of the mass of an atom of C¹²
Page 22 :
GRAM MOLECULAR MASS, , When molecular mass of the substance is expressed in gram, then the value becomes gram molecular mass.
Page 23 :
ATOMICITY, , Total number of atom in a molecule of elementary substance, is called atomicity
Page 24 :
ACTUAL MASS, , The mass of one atom or one molecule of a, substance is called as actual mass., Ex. (i) Actual mass of O2 = 32 amu = 32 × 1.67 × 10–24 g ® Actual, mass, (ii) Actual mass of H2O = (2 + 16) amu = 18 × 1.67 × 10–24g = 2.99 ×, 10–23 g
Page 25 :
The modern atomic weight scale is based on., (1) C12 (2) O16 (3) H1 (4) C13, , The actual molecular mass of chlorine is :, (1) 58.93 × 10–24 g (2) 117.86 × 10–24 g, , (3) 58.93 × 10–24 kg (4) 117.86 × 10–24 kg
Page 26 :
PERCENTAGE FORMULA (% BY MASS), , Number of atom (Atomicity) × atomic mass, mass % of an element, , = _____________________________________, Molecular mass, , × 100
Page 27 :
MOLECULAR FORMULA, , The molecular formula of a compound represents the actual number of atoms, present in 1 molecule of the compound., i.e. it shows the real formula of its 1 molecule.
Page 28 :
EMPIRIC AL FORMULA, , The empirical formula of a compound express the simplest whole number ratio, of atoms of various elements present in 1 molecule of the compound.
Page 29 :
RELATIONSHIP BETWEEN EMPIRIC AL &, MOLECULAR FORMULA, Molecular Formula = n × Empirical Formula, [Where n = natural no. (1, 2, 3,.........)], Molecular formula mass, or n =, , ____________________, ., , Empirical formula mass
Page 31 :
In a compound x is 75.8% and y is 24.2% by weight present. If atomic weight of x and, y are 24 and 16 respectively. Then calculate the empirical formula of the compound.
Page 33 :
In a compound Carbon is 52.2%, Hydrogen is 13%, Oxygen is 34.8% are present, and molecular mass of the compound is 92. Calculate molecular formula of the, compound ?