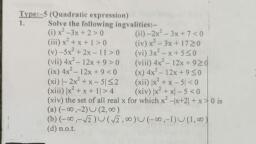Page 1 :
a, 13., , 14., , 15., , 16., , 17., , 18., , 19., , 20., , 21., , 22., , Which of the following is formed when zine reacts with, sodium hydroxide?, , (@) Hydrogen gas (b) Sodium zineate, , (© Zine oxide (@)_ Both (a) and (b), Identify x and y in following reaction. What is the mixture, of x and y called?, , , , CH,(g) + H,O(g) BX x + y, (@ x= CO,, y= HO, water gas, (b) x= CO, y= H,O, syn gas, (©) x= CO, y=H,, water gas, (@) x= CO, y= H,, syn gas, , , , Why is water gas (mixture of CO and H,) also called ‘syn, , gas’?, , (a) Because it is synthesised from sewage, saw — dust,, , 1p wood ete., , (b) Because it is synthesised from methane gas, , (©) Because it is used in the synthesis of methanol and, a number of hydrocarbons,, , (@)_ None of these, , Which of the following statements is correct?”, , (a) Production of syngas from coal is called coal, gasification., , (b) CO(g) + H,O (g)—atia? CO(g) + Hy (g), represents water gas shift reaction, , (© CO, formed in water gas shift reaction is removed by, scrubbing with sodium zincate solution, , (@)_ Both (a) and (b), , Which one of the following pairs of substances on reaction, , will not evolve H, gas?, , (@)_ Iron and H,SO, (aqueous), , (b) Ironand steam, , (©) Copper and HCI (aqueous), , (@)_ Sodium and ethyl aleohol, , Which ofthe following metal evolves hydrogen on reacting, with cold dilute HNO, ?, , , , Tatas, , @ Mg () Al, (© Fe @ cu, Hydrogen is evolved by the action of cold dil. HNO on, (a) Fe (b) Mn, (©) Cu (@ Al, , In Bosch’s process which gas is utilised for the production, of hydrogen gas ?, , (@) Producer gas (b) Water gas, , (© Coal gas (@_ None of these, , Hydrogen is not obtained when zine reacts with, , (@) Cold water () dil HCL, , © dil. H,SO, (@ HotNaOH 20%), Which one of the following pairs of substances will not, produce hydrogen when reacted together?, , (@) Copper and cone. nitric acid, , (b) Ethanol and metallic sodium, , (c) Magnesium and steam, , (@)_ Phenol and metallic sodium, , 23., , 24,, , 25., , 26., , 27., , 28., , 29., , 30., , eee, , Very pure hydrogen (99.9) can be made by which of the, , following processes ?, , (a) Reaction of methane with steam, , (b) Mixing natural hydrocarbons ofhigh molecular weight, , (©) Electrolysis of water, , (4) Reaction of salts like hydrides with water, , Which of the following is formed on reaction of carbon, , monoxide gas with dihydrogen in presence of cobalt as, , a catalyst?, , (2) Methanal (6) Methanol, , (©) Methane (@) Formic acid, , Which of the following is not a use of dihydrogen ?, , (a) It used in fuel cells for generating electrical energy,, , (b) Atomic hydrogen and oxy-hydrogen torches are, used for cutting and welding purposes., , (©) Itused in the synthesis of hydroquinone and tartaric, acid, , (@) Both (b) and (c), , Elements of which of the following group do not form, , hydrides?, , (a) Alkali metals (b) Halogens, , () Alkaline earth metals (d) Noble gases, , Which of the following statements is incorrect?, , (a) Ionic hydrides are stoichiometric compounds of, dihydrogen formed with most of s-block elements, , (b)_ Ionic hydrides are crystalline, non-volatile and nonconducting in solid state., , (©) Melts of ionic hydrides conduct electricity and, liberate dihydrogen gas at cathode, , (d) Both (a) and (c), , Saline hydrides react explosively with water, such fires can, , , , , , be extinguished by, (a) water (b) carbon dioxide, (©) sand (@)_ None of these, , Choose the correct option for following hydrides., B,H,, CH,, NH, and HF, (a) Electron deficient hydride = B,H, and HF, Electron precise hydride = CH,, Electron rich hydride = NH,, (b) Electron deficient hydride = B)H,, Electron precise hydride = CH,, Electron rich hydride = NH, and HF, () Electron deficient hydride = CH,, Electron precise hydride = BH,, Electron rich hydride = NH, and HF, (@)_ Electron deficient hydride = CH, and HF, Electron precise = B,H,, Electron rich hydride = NH,, Elements of which of the following group(s) of periodic, table do not form hydrides, (a) Groups7,8,9 (b) Group 13, (©) Groups15,16,17 (d)_ Group 14, bs : an