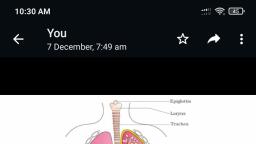
Page 1 :
Important property of liquids, Vapour pressure, , It is defined as the pressure exerted by the, vapour in equilibrium with its own liquid., , It depends on the nature of the liquid and, the temperature. As the temperature, increases, the vapour pressure also, increases., , Boiling point, , At a particular temperature, the vapour, pressure becomes equal to atmospheric, pressure. This temperature is called, boiling point., , The boiling point at 1 atm pressure is, called normal boiling point, The boiling point at 1 bar pressure is called
Page 2 :
standard boiling point, , Q)Give a reason for the use of pressure, cooker to cook food, at high altitudes., , At high altitudes, the atmospheric pressure, is low. So liquids boil at lower, temperatures than at sea level and hence a, pressure cooker is used for cooking food., , Surface tension, , It is defined as the force acting per unit, length perpendicular to the line drawn on, the surface of liquid. It is denoted by Greek, letter y (Gamma). Its SI unit is Nm-1, , Q)Liquid drops assume spherical shape., Why?, , Every liquid tries to reduce their energy by, decreasing the surface area. For a given
Page 3 :
volume sphere has the minimum surface, area. So liquid drops assume spherical, shape., , Q)Sharp glass edges are heated for, making them smooth.Give the reason?, On heating, the glass melts and the, surface of the liquid tends to take the, rounded shape at the edges, due to, surface tension., , Q)ldentify the property of liquid behind the, capillary rise.?, Ans: Surface tension, , Q)Which property of liquids is associated, , with fire polishing of glass?, Ans :Surface tension, , Viscosity
Page 4 :
Viscosity is a measure of internal, resistance offered by the different layers, of a liquid., , Q) The glass window panels of old, buildings are thicker at the bottom than at, the top?, , Glass is a very viscous liquid. So it has a, tendency to flow very slightly. Hence the, window panels of old buildings are thicker, at the bottom than at the top., , Q Why viscosity of liquid decrease with, temperature?, , Viscosity of liquids decreases as the, temperature rises because at high, temperature, molecules have, , high kinetic energy and can overcome the, intermolecular forces.