Page 1 :
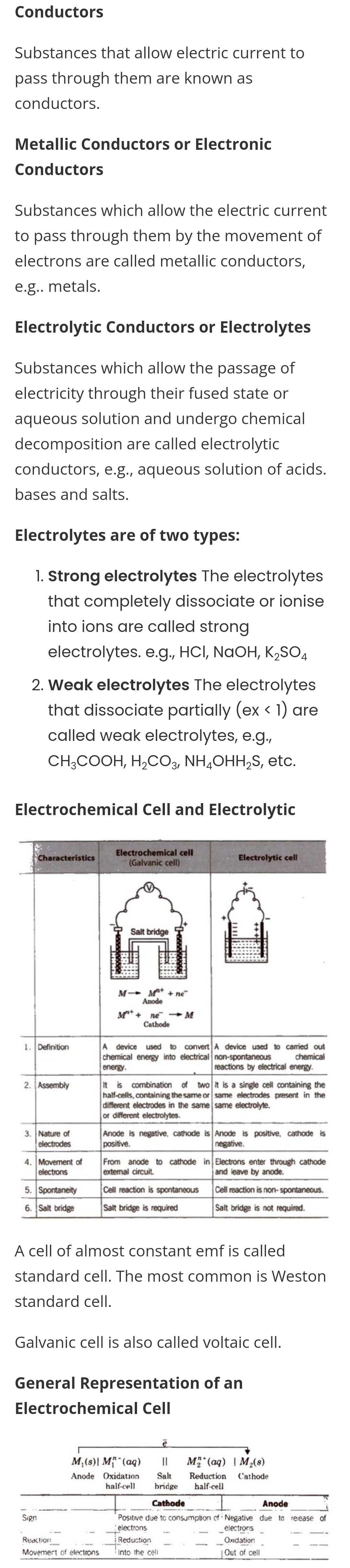
Conductors, , Substances that allow electric current to, pass through them are known as, conductors., , Metallic Conductors or Electronic, Conductors, , Substances which allow the electric current, to pass through them by the movement of, electrons are called metallic conductors,, e.g.. metals., , Electrolytic Conductors or Electrolytes, , Substances which allow the passage of, electricity through their fused state or, aqueous solution and undergo chemical, decomposition are called electrolytic, conductors, e.g., aqueous solution of acids., bases and salts., , Electrolytes are of two types:, , 1. Strong electrolytes The electrolytes, that completely dissociate or ionise, into ions are called strong, electrolytes. e.g., HCl, NAOH, K,SO,, , 2. Weak electrolytes The electrolytes, that dissociate partially (ex < 1) are, called weak electrolytes, e.g.,, CH3;COOH, H2CO3, NH,OHH,ZS, etc., , Electrochemical Cell and Electrolytic, , From anode to cathode in| Electrons enter through cathode, extemal circuit., . | Spontaneity Cell reaction is spontaneous _| Cell reaction is non- spontaneous., 6. |Salt bridge Salt bridge is required i L, , , , , , A cell of almost constant emf is called, standard cell. The most common is Weston, standard cell., , Galvanic cell is also called voltaic cell., , General Representation of an, Electrochemical Cell, , M\(s)i M?"(aq) sil Ss MB*(ag) | Mx(s), Anode Oxidation Salt Reduction Cathode, half-cell bridge half-cell, , , , , , St eee ence, Positwe due tc consumption cf' Negative due to release of, __jelectrons se _. _electrons _, , __jReduction _ _ ___ Oxidation _ ame, , Movemert of elections ‘Into the celi Out of cell, , Sign, , Reaction