Page 4 :
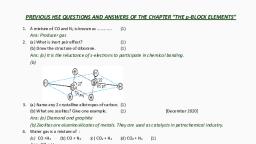
(3) [March 2018], , O, Ans: X is CH3-CH=CH2, Y is CH3-CH, O, , CH2 and Z –isCH3-CHO, O, , 19. a), , Cyclopentadienyl anion is aromatic. Why?, (1), b) Explain the following reactions:, i) Substitution ii) Addition, c) Ethyne is acidic in nature. Explain., (2), [July 2017], , (2), , Ans: a) This compound is aromatic since it contains 6 delocalised π electrons according to Huckel’s rule., b) i) Substitution Reaction: It is the replacement of an atom or group of atom by another atom or atom, group. E.g.: Halogenation CH4 + Cl2 hυ, CH3Cl + HCl, ii) Addition Reaction: It is the process of addition of simple molecules like H2, X2, HX etc. to an, unsaturated system., CH2 = CH2 + HBr → CH3-CH2Br, c) In Ethyne, the H atoms are attached to sp hybridized carbon atoms. Due to the greater s-character, and electronegativity of sp hybridized C, it attracts the electron pairs of C – H bonds strongly. So the, hydrogen atom is readily removed as H+ and hence ethyne is acidic., 20. Benzene and benzeniod compounds show aromatic character., a) Select the aromatic compounds from the following:, , b) Suggest a method to convert ethyne to benzene. (2), c) Give the products formed when benzene reacts with the following:, i), CH3Cl/AlCl3, ii) Cl2/hν, [March 2017], , Ans: a), , b) 3 C2H2 Red hot iron tube & 873K, c) i) Toluene, ii) Chlorobenzene, , C6H6, , 21. a) i) Complete the following reactions:, , Hydrocarbons - Prepared by ANIL KUMAR K L, GHSS ASHTAMUDI, KOLLAM, Join Telegram Channel, https://t.me/hsslive, , Prepared by Anil Kumar K L, , Downloaded from www.Hsslive.in ®, , Page 4
Page 5 :
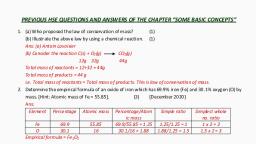
ii) Write the names of the above reactions?, (2), b) Baeyer’s reagent is used to find whether the compound is unsaturated or not. What is Baeyer’s reagent? (1), c) What is the product formed when ethylene is treated with Baeyer’s reagent? (2), [September 2016], , Ans: a) i), 1) CH3-CH2-CH2-CH3, 2) C6H5-CH3, ii), 1) Kolbe’s Electrolysis, b) Cold dilute aqueous KMnO4, c) Ethylene glycol, , 2) Friedel Craft’s reaction, , 22. a) 1-Alkynes are weakly acidic in nature. Give any two reactions to show the acidic character of ethyne. (2), b) From the following, select the one in which Markownikoff’s rule is best applicable., i) C2H4 + HCl, ii) C3H6 + Br2, iii) C3H6 + HBr iv) C3H8 + Cl2, (1), c) Hydrocarbons exhibit isomerism., i) Name the type of isomerism exhibited by 2-Butene., ii) Draw the structure of the isomers of 2-butene and select the one which is more polar. (2) [March 2016], , Ans: a) CH≡CH + Na → CH≡C-Na+ + ½ H2, CH3-C≡CH + Na → CH3-C≡C-Na+ + ½ H2, b) iii) C3H6 + HBr, c) i) Geometrical isomerism, ii), , Cis-But-2-ene is more polar than the trans form., 23. Controlled oxidation of alkanes in the presence of suitabie catalysts give a variety of products., a) Complete the following reaction :, CH4 + O2 Mo2O3/heat, ………………. + H2O (1), b) Free rotation about a carbon-carbon single bond is permitted in an alkane molecule., What are conformers? Draw the structure of the eclipsed and staggered conformers of ethane in Sawhorse, and Newman projections and explain their relative stability. (4), [October 2015], , Ans: a) HCHO, Hydrocarbons - Prepared by ANIL KUMAR K L, GHSS ASHTAMUDI, KOLLAM, Join Telegram Channel, https://t.me/hsslive, , Prepared by Anil Kumar K L, , Downloaded from www.Hsslive.in ®, , Page 5
Page 6 :
b) The different spatial arrangements of atoms arising due to free rotation around a C-C single bond, are called conformations., For projection formulas refer Question no. 4 and 7., Staggered conformation is stabler than eclipsed form due to minimum repulsive forces between the, electron clouds of C-H bonds., 24. Write the IUPAC names of the following compounds:, , Ans: a) Pent-4-en-2-ol, b) 2,5-Dimethylheptane, 25. a) Complete the following chemical equations:, (1), (1), , (1), b) Explain the geometrical isomerism taking 2-Butene as an example., , (2), , [March 2015], , Ans: a), i), CH3-CH2-CH2-CH3, ii), CH2=CH2, iii), C6H5-CH3 (Toluene), b) Refer the answer of the question number 2., 26. a) Draw the cis and trans isomers of the following compound:, C2H5-C(CH3) = C(CH3)-C2H5., (2), b) Complete the following reactions., (1), i., 3CH ≡ CH Red hot iron tube at 873 K, …………, ii., CaC2 + 2H2O, Ca(OH)2 + …………., c) Draw the sawhorse projections for eclipsed and staggered forms of an ethane molecule., , Ans: a) 5H2C, , C2H5, , 5H2C, , C=C, 3HC, , (2), , CH3, C=C, , CH3, , 3HC, , C2H5, Trans-isomer, , Cis-isomer, b) i) C6H6 (Benzene), ii) C2H2 (Ethyne or acetylene), c) Refer the answer of the question number 7., , 27. a) How is alkane prepared by Kolbe’s electrolytic method?, (2), b) Select the activating groups from the following:, (1), i) –NH2, ii) –SO3H, iii) –CH3, iv) –COOH, Hydrocarbons - Prepared by ANIL KUMAR K L, GHSS ASHTAMUDI, KOLLAM, Join Telegram Channel, https://t.me/hsslive, , Prepared by Anil Kumar K L, , Downloaded from www.Hsslive.in ®, , Page 6
Page 8 :
b) Draw Newman’s projections of the two conformers of ethane. Which among these is more stable? Justify. (2), c) An alkene on ozonolysis followed by reduction of the ozonide formed with zinc and water gave a mixture of, ethanal and methanal., i), Identify the alkene., (1), ii), Illustrate the above mentioned reaction using the chemical equation., (1), [March 2013], , Ans: a) Torsional strain, b) Refer the answer of the question number 4., Staggered conformation is stabler than eclipsed form due to minimum repulsive forces between the, electron clouds of C-H bonds., c) i) Propene, ii) Refer the answer of the question number 5., 31. a) Name the following reactions:, i), C6H14, Anhydrous AlCl3/HCl, n-hexane, ii), , C6H14, , CH3 – CH – CH2 – CH2 – CH3, CH3, 2-Methyl pentane, , V2O5/773K, 10 – 20 atm, , Benzene, C4H8 + C2H6, butene ethane, (3 x 1 = 3), b) Naphthalene is an aromatic compound. Explain its aromaticity using Huckel’s rule. (2) *September 2012+, iii), , C6H14, , 773K, , Ans: a) i) Isomerisation, ii) Aromatisation, iii) Pyrolysis, b) According to Huckel’s rule, cyclic, planar, conjugated systems containing (4n+2) π electrons are, aromatic. Naphthalene contains 10 π electrons and follows this rule. So it is aromatic., 32. Hydrocarbons are organic compounds containing carbon and hydrogen only., a) Complete the following chemical reactions:, i), 2CH3Br + 2 Na dry ether …………… + 2 NaBr, ii), …………… + Zn heat C6H6 + ZnO, iii), + 3Cl2 UV, 500K, …………………. (3 x 1 = 3), b) Analyze the following reaction:, CH3 – CH = CH2 + H – Br, ‘A’ + ‘B’, If ‘A’ is the major product and ‘B’ is the minor product, identify ‘A’ and ‘B’. Also name the related rule. (2), [March 2012], , Ans: a), i), CH3 – CH3 (Ethane), ii), C6H5-OH (Phenol), iii), Benzene hexachloride or,, , Hydrocarbons - Prepared by ANIL KUMAR K L, GHSS ASHTAMUDI, KOLLAM, Join Telegram Channel, https://t.me/hsslive, , Prepared by Anil Kumar K L, , Downloaded from www.Hsslive.in ®, , Page 8
Page 10 :
Here 2-bromopropane is the major product., 35. In a special condition, addition of HBr to unsymmetrical alkene takes place contrary to Markovnikov’s rule., a) What is the special condition?, (1), b) Give the mechanism of anti Markovnikov’s addition of HBr to propene., (4), [September 2010], , Ans: a) The special condition is the presence of organic peroxide., b) Mechanism of anti Markovnikov’s addition, , 36. a) The spacial arrangements of atoms which can be converted into one another by rotation around a C – C, single bond are called conformations., i), Represent Sawhorse and Newman projection formulae of staggered and eclipsed conformations of, ethane., (2), ii), Compare the stabilities of staggered and eclipsed conformations., (1), b) Consider the reaction given below:, CH3 – CH = CH2 + HBr, CH3 – CHBr – CH3 + CH3 – CH2 – CH2Br, i), Identify the major product obtained. (1), ii), Name the rule governing the formation of the major product. (1), [March 2010], , Ans: a), i), Refer the answer of the question number 4 and 7., Hydrocarbons - Prepared by ANIL KUMAR K L, GHSS ASHTAMUDI, KOLLAM, Join Telegram Channel, https://t.me/hsslive, , Prepared by Anil Kumar K L, , Downloaded from www.Hsslive.in ®, , Page 10
Page 11 :
ii), , Staggered conformation is stabler than eclipsed form due to minimum repulsive forces between, the electron clouds of C-H bonds., b) i) CH3 – CHBr – CH3 (2-Bromopropane), ii) Markovnikov’s rule., 37. a) How will you prepare ethane by Kolbe’s electrolytic method?, (2), b) Expalin the Markovnikov’s rule for the addition reaction using a suitable example., , (3), , [March 2009], , Ans: a) By the electrolysis of aqueous solution of sodium or potassium acetate., 2CH3COONa + 2H2O, CH3 – CH3 + 2CO2 + 2NaOH + H2, Sod. Acetate, Ethane, b) Refer the answer of the question number 28 (b), 38. a) Consider the reaction between benzene and nitrating mixture., + HNO3 Conc. H2SO4, , ?, , (1), , b) What is the reacting species in the above reaction? (1), c) How is the species formed in the system? (1), [[June 2008], , Ans: a), , b) Nitronium ion (NO2+), c) It is produced by the transfer of a proton from H2SO4 to HNO3 followed by dehydration of the, resulting product., #############################################################################, , Hydrocarbons - Prepared by ANIL KUMAR K L, GHSS ASHTAMUDI, KOLLAM, Join Telegram Channel, https://t.me/hsslive, , Prepared by Anil Kumar K L, , Downloaded from www.Hsslive.in ®, , Page 11