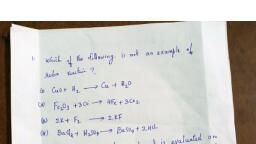Page 1 :
Join Telegram Channel: https://t.me/hsslive, Downloaded from www.Hsslive.in ®, XII CHEMISTRY (WITH MALAYALAM), CHAPTERS 1 TO 16, (BASED ON FOCUS POINTS 2022), PREPARED BY: YOOSAFALI T K, GHSS VARAVOOR, 9947444175, YOUTUBE CHANNEL: CHEM DSM, CHAPTER 1, 1. What are differences between crystalline solids and amorphous solids?, Crystalline solids, Amorphous solids, In amorphous solids, particles do not have the, orderly arrangement, They have short range order, They do not have sharp melting point, In crystalline solids, particles are arranged in, ordered manner., They have long range order, They have sharp melting point, They have definite heat of fusion, They can be cleaved along definite, Crystalline solids are anisotropic, They do not have definite heat of fusion, They undergo irregular cleavage on cutting., Amorphous solids are isotropic., SS OR0444175, Crystalline solids are true sólids, Amorphous solids are pseudo solids, Eg. Nacl, KCI, sugar, diamond, graphite, marble,, naphthalene, copper, benzoic acid, KNO3 ,quartz, Eg. Glass, Plastic(Polyurethane, Teflon,, cellophane, poly vinyl chloride) ,Rubber, pitch, They have long range order, They have short range order, NGAJBROMŮ emuɔglnwjavð pseudo solids mm, Gmmɔglnwlaud ( true solids) ei, 263:-NaCl, KCI, KNO3, n16 mɔo, No,, Osengand, amucejoannwlnd , PVC) noy8,
Page 2 :
Join Telegram Channel: https://t.me/hsslive, Downloaded from www.Hsslive.in ®, 2. Anisotropic :- In Crystalline solids , the physical properties such as electrical conductivity, thermal, conductivity, refractive index etc. are different in different directions, Anisotropic :- (8 ne emɔgluagl BUmla nJomBBIW MɔnJ alelam,, 3. Isotropic:- In Amorphous solids , the physical properties such as electrical conductivity, thermal, conductivity, refractive index etc. are same in all directions, 4. Why amorphous solids are considered as pseudo solids or super cooled liquids?, Some of the amorphous solids like glass have a tendency to flow very slowly. So they, considered as, pseudo solids or super cooled liquids., Example: The glass panes of windows of old buildings are found to be slightly thic, at the bottom than, at the top. This is because the glass flows down very slowly and makes the bottom portion slightly, thicker., 5. What are crystal lattice (space lattice) and unit cells?, The three dimensional arrangement of constituent particles in space is called crystal lattice (space, lattice)., Smallest repeating portion of the crystal lattice is called unit cell., A unit cell is characterized by edge lengths (a, b,c) and axial angles(a, B, y), elɔglmi (müenimů eloglmi)., eloglmi ), OLANIA2,OS mignno [edge lengths (a , b, c)] eOlwoi eoong}A2}o[axial angles(a, B, y)], 6., Which are seven types of crystal systems and give their bond parameters, Axial angles, a = B = y = 90 °, a = B = y = 90, a = B = y = 90 °, a = B = 90 ° y 120, a = B = y + 90 °, a = y = 90 ° B + 90 °, a + B + y + 90 °, Crystal systems, Edge lengths, Cubic, a = b = c, Tetragonal, a = b *c, Orthorhombic, a * b + c, a = b +c, Нехаgonal, Rhombohedral or Trigonal, a = b = c, a + b *c, a * b *c, Monoclinic, Triclinic
Page 3 :
Join Telegram Channel: https://t.me/hsslive, Downloaded from www.Hsslive.in ®, 7. Match box is orthorhombic type. Give its bond parameters, arb+c, α= β=γ=90, 8. Explain primitive (simple) and centered cubic unit cells., Simple (primitive) cubic,, Body centred cubic (bcc),, Face centred cubic (fcc)., In simple (primitive) cubic unit cells, the particles are present only at the corners, In body centered cubic unit cells, the particles are present at the corners and at the centre of the unit, (i), the unit cells., (ii), cells., (iii), In face centered cubic unit cells, the particles are present at the corners and at the face centre of the, unit cells, (i), mlmnuð ay6mlanlo(Primitive cubic) nS) 0elaglo nɔmcn Amlaaưð, (ii), (ii), 9., Contribution of each atom at the corner 1/8, Contribution of each atom at the face +1/2, Contribution of each atom at the edge 1/4,, Contribution of each atom within the body->1, 10. Calculate the number of particles in 3 types cubic unit cells., Types of unit cells, Position of, Body centre, contribution contribution, Corner, Face, Total number of, particles, 8 corners, contribution, particles, Simple cubic, 1, -X 8 =1, 1, 8., 1, Body centred, 8 corners+, X8%=D1, 1, 2, 8, cubic, 1 body centre, 1, Face centred cubic 8 corners +, -X 8 =1, 4, ;X 6 =3, 8, 2, 6 face centres, Simple cubic = Gx 8) = 1, %3D, Body centred cubic = (- x8) + 1 = 2, x 8) + (; x6), Face centred cubic =, = 1+3 = 4
Page 4 :
Join Telegram Channel: https://t.me/hsslive, Downloaded from www.Hsslive.in ®, 11. What are Defects or imperfections in solids?, Any deviation from perfectly ordered arrangement in a crystal is called defects or imperfections., Defects are point defects, line defects and plane defects., 12. What are Point defects ?, If the imperfection is near few atoms are called Point defects., These are two types (1) Stoichiometric defects (11) Non stoichiometric defects., (1), (1I), 13. What are Stoichiometric defects?, The defects do not disturb the stoichiometry of the compound are called stoichio metric defects., The stoichio metric defects shown by ionic solids are Schottky defects and Frenkel defects., (1), (II), 14. What are differences between Schottky defects and Frenkel defects?, Schottky defects, Frenkel defects, B, 9SSMVOC474445, B, B-, B-, It arises by the missing of equal number of cations and, It arises by the movement of cations from the lattice, anions from the lattice site, site to the interstitial site., Density decreases, Found in ionic compounds with high co-ordination, Density remains same., Found in ionic compounds with low co-ordination, number and are in similar size of cations and anions, number and cations are smaller than the anions, Eg:-NaCl,KCI, CsCI, Eg:-AgCI, Zns, eioglmi nomglmd mimo me mo, MUIIBM (Density ) nWm, maBm (Density) 0ɔn3m1
Page 5 :
Join Telegram Channel: https://t.me/hsslive, Downloaded from www.Hsslive.in ®, 260:-NaCl, KCI, CsCI, 260:-AgCI, ZnS, 15. AgBr shows both schottky defect and Frenkal defect., 16. What are Non- stoichiometric defects?, The defects which disturb the stoichiometry of the compound are called non- stoichio metric defects., These are two types, (1), Metal excess defect (due to anion vacancies, due to the presence of extra interstitial cations), (I1), Metal deficiency defects., (1), (II), 17. Metal excess defect due to anion vacancies :-((When sodium chloride crystal is heated in the presence of, sodium vapour, the colour of the crystal turns yellow. Why?), When sodium chloride is heated in the presence of sodium, vapour, some of the sodium atoms deposit on the surface of the, crystal. The chloride ion diffuse to the surface and combine with, sodium atoms, which become ionized by losing electrons. These, electrons diffuse back to the crystal and occupy the anionic vacant, sites created by chloride ions. The anion sites occupied by, electrons are called F-centres (colour centres). These F-centres, are responsible for the colour of the compound. The colour results, by the excitation of these electrons when they absorb energy, B., from the visible light falling on the crystals., (Similarly potassium in potassium chloride makes it violet and lithium in lithium chloride makes it pink), DelasCIsImd ensoBlw1siee mm nomuglamF-centres (colour centres) am