Page 1 :
Battery Chemistry:Batteries:, , When two or more electrochemical cells are electrically interconnected, each of which, containing two electrodes and an electrolyte is called a Battery., , Batteries are classified into a two categories depending on their recharging capabilities., Primary Batteries: “These are non-rechargeable and are meant for single use and to be, discarded after use”., , These are non-reversed and are less expensive and are offer used in ordinary gadgets like, torch lights, watches and toys., Eg: Leclanche cell, Dry cell., , Secondary Batteries: - These are rechargble and are meant for multi cycle use. After every us, the electrochemical reaction could be reversed by external application fades or lost due to, leakage or internal short circuit. Eg: Lead-acid cell, Ni/ed cell
Page 2 :
Differences between Primary and secondary batteries:, , , , Primary cells, , Secondary cells, , , , , , 1. These are non-rechargeable and, meant for a single use and to be, discarded after use., , Cell reaction is not reversible., Cannot be rechargeable., , Less expensive., , Can be used as long as the, materials are active in their, composition., , Eg: Leclanche cell, ‘Li’ Cells., , veer, , , , 1. These are rechargeable and meant, for multi cycle use., , Cell reaction can be reversed., Can be rechargeable., , Expensive., , Yen, , 5. Can be used again and again by, , recharging the cell., Eg; Lead- acid cell, Ni-cd cells.
Page 3 :
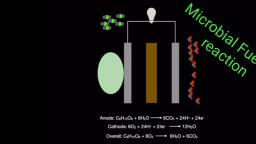
Fuel Cell, , Fuel cell is an electrochemical cell which converts chemical energy, contained in readily available fuel oxidant system into electrical energy., , Fuel cell is that the fuel & oxidant are stored outside the cell. Fuel and, Oxidant are supplied continuously and separately to the electrodes at which they, undergo redox reactions., , Eg: H,-O, fuel cell, Methanol -O, fuel cell, , Fuel + Oxidant > Oxidation Products + electricity
Page 4 :
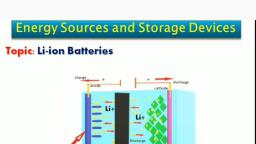
Batteries, , A battery is a storage device used for the storage, of chemical energy and for the transformation of chemical, , energy into electrical energy., , Types of Batteries:, , » Primary Batteries (or) Non Reversible Battery, Ex: Lithium cell, Leclanche cell, , » Secondary Batteries (or) Reversible Battery, lead-acid. Ni-Cad Batteries, Lithium-ion Batteries, , >» Fuel Cells, Ex: Hydrogen-oxygen fuel cell.