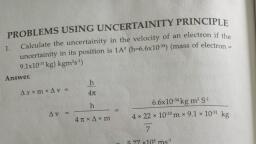Page 1 :
MODULE 3, CO3 Explain various engineering materials for domestic and industrial applications, M3.02 Classify the types of polymers and its applications in daily life., Syllabus, Polymers – monomer, polymerization, classification- homo and copolymers, addition and, condensation polymer, common polymers – polythene, PVC, Nylon-66 and Bakelite monomers and uses, thermoplastics and thermosetting plastics- with one example each., Natural rubber, vulcanization of rubber, properties of vulcanized rubber. Synthetic, rubberBuna S, Buna-N – Monomers, POLYMER, The word ‘polymer’ is coined from two Greek words: poly means many and mer means unit, or part. The term polymer is defined as a very large molecule having high molecular mass,, which are formed by joining of repeating structural units on a large scale. The repeating, structural units are derived from some simple and reactive molecules known as monomers, and are linked to each other by covalent bonds., Polymerisation: The process of formation of polymers from respective monomers is called, polymerisation., Examples, 1. Polyethene formed from ethylene monomers, , 2. Polyvinyl chloride (PVC) formed from vinyl chloride monomers, , 1, , Chemistry Notes, GPC Cherthala
Page 2 :
CLASSIFICATION OF POLYMERS, I) Based on source polymers are classified into three., a) Natural polymers: Polymers which are found in nature, i.e; in plants and animals., Examples: Cellulose, Natural Rubber, Starch, Proteins, b) Synthetic polymers: These are man made polymers, Examples: Polythene, Polypropene, PVC, Teflon, Buna-S, Buna-N, c) Semisynthetic polymers: Polymers which are derived from natural polymers by, chemical modification., Example: Cellulose acetate, Cellulose nitrate (gun cotton), II) Based on the nature of monomers, polymers are classified into two., a) Homopolymer: Polymer which is made up of only one type of monomers., Examples, 1. Polyethene formed from ethylene monomers, , 2. Polyvinyl chloride (PVC) formed from vinyl chloride monomers, , 2, , Chemistry Notes, GPC Cherthala
Page 3 :
b) Co-Polymer: Polymer which is made up of two or more types of monomers., Examples:, 1., , Buna-S formed from 1,3-Butadiene and Styrene, , 2., , Nylon 6,6 formed from hexamethylene diamine and adipic acid, , III) Based on the mode of synthesis, polymers are classified into two., a) Addition polymers: The addition polymers are formed by the repeated addition of, monomer molecules possessing double or triple bonds. In these polymers, the, molecular formula of the repeating unit is the same as that of the monomer., Examples, 1. Polyethene formed from ethylene monomers, , 2. Polyvinyl chloride (PVC) formed from vinyl chloride monomers, , 3, , Chemistry Notes, GPC Cherthala
Page 4 :
b) Condensation Polymers: The condensation polymers are formed by repeated, condensation reaction between two different bi-functional or tri-functional, monomeric units. In these polymerisation reactions, the elimination of small, molecules such as water, alcohol, hydrogen chloride, etc. take place. In these, polymers, the molecular formula of the repeating unit is not the same as that of the, monomer., Examples, 1. Nylon 6,6 formed from hexamethylene diamine and adipic acid, , 2. Terylene (Dacron) formed from Terephthalic acid and Ethylene glycol, , 4, , Chemistry Notes, GPC Cherthala
Page 5 :
Differences between addition polymers and condensation polymers, ADDITION POLYMERS, , CONDENSATION POLYMERS, , Formed by repeated addition of monomers Formed by repeated condensation of two, containing double bond or triple bond, different bi-functional or tri-functional, monomers, No elimination of small molecules, Elimination of small molecules such as, water, alcohol, HCl etc. take place, Molecular formula of the repeating unit of Molecular formula of the repeating unit of, the polymer is the same as that of the the polymer is not the same as that of the, monomer, monomer, Formed by chain growth polymerisation, Formed by step growth polymerisation, Examples: Polythene, PVC, Examples: Nylon 6,6 , Terylene, , PLASTICS, Plastics are polymers with the capability of being moulded or shaped. The name, "plastic" refers to the property of plasticity, the ability to deform without breaking., The word, plastic, was derived from the word ‘Plastikos’ meaning ‘to mould’ in Greek., THERMOPLASTICS AND THERMOSETTING PLASTICS, Based on the behaviour when heated, plastics are classified into two, thermoplastics, and thermosetting plastics., Thermo plastics, The polymers which can be repeatedly made soft and hard by heating and, cooling are called thermoplastics. These polymers possess intermolecular forces of, attraction intermediate between elastomers and fibres. These are linear or slightly, branched polymers. They can be remoulded., Examples: Polythene, PVC, Polystyrene, Thermosetting plastics, These are cross linked or heavily branched polymers, which on heating undergo, extensive cross linking in moulds and again become infusible. They can be moulded, only once. They cannot be reused., Examples: Bakelite, urea-formaldehyde resin, 5, , Chemistry Notes, GPC Cherthala
Page 6 :
Differences between thermoplastics and thermosetting plastics, Thermoplastics, Formed by addition polymerisation, Linear structure, It becomes soft on heating and hard on, cooling, Soft, weak and less brittle, Soluble in organic solvents, It can be remoulded, Recyclable plastics, Examples: Polythene, PVC, , Thermosetting plastics, Formed by condensation polymerisation, Three-dimensional network structure, Does not become soft on heating, Hard, strong and more brittle, Insoluble in organic solvents, It cannot be remoulded, Non-recyclable plastics, Examples: Bakelite, Urea-formaldehyde, resins, , NATURAL RUBBER, Rubber is a natural polymer and possesses elastic properties. It is manufactured from, rubber latex which is a colloidal dispersion of rubber in water. This latex is obtained, from the bark of rubber tree. Natural rubber may be considered as a linear polymer, of isoprene (2-methyl-1, 3-butadiene)., , Limitations of Natural rubber, Natural rubber becomes soft at high temperature (above 335 K) and brittle at low, temperatures (below 280K), Shows high water absorption capacity, Soluble in non-polar solvents, Non-resistant to attack of oxidising agents, In order to overcome these limitations, vulcanisation is carried out., , 6, , Chemistry Notes, GPC Cherthala
Page 7 :
VULCANISATION, The process of heating natural rubber with sulphur in presence of ZnO at a, temperature range between 373 K to 415 K is called vulcanization. On vulcanization,, sulphur forms cross links at the reactive sites of double bonds and thus the rubber, gets stiffened. These cross links make the rubber hard, strong and remove the, tackiness of rubber. The extend of hardness depends on the amount of rubber added., Differences between natural rubber and vulcanised rubber, Natural Rubber, , Vulcanised Rubber, , Soft, , Hard, , Less elastic, , More elastic, , Non-heat resistance, , More heat resistance, , Low melting point, , High melting point, , Easily oxidised, , Resist oxidation, , Merits of Vulcanisation, 1. Vulcanisation increases tensile strength, elasticity and extensibility., 2. Vulcanised rubber possesses low water absorption tendency., 3. Vulcanised rubber has higher resistance to oxidation, abrasion and wear and tear., 4. Vulcanised rubber is a better electrical insulator., 5. It increases resistance to organic solvents, fats and oils., 6. It makes rubber less sensitive to temperature changes., SYNTHETIC RUBBER, A synthetic rubber is a man-made rubber like polymer which possesses elastomeric, properties. The monomers are usually obtained from petroleum. Synthetic rubber also, has many industrial applications similar to natural rubber; in the field of the automotive, industry for tires, hoses, belts, flooring, doors, and windows., Examples: Neoprene, Buna-S, Buna-N, , 7, , Chemistry Notes, GPC Cherthala
Page 8 :
Synthetic rubber, , Monomers, , Uses, , Buna – S, , 1.3-Butadiene and Styrene, , 1. For making tyres, 2. For making rubber tubes and, hoses, 3. Conveyer belts, 4. Shoe heals and foot wares, , Buna – N, , 1,3-Butadiene and Acrylonitrile, , 1. For making oil seals, 2. Manufacture of hoses, 3. For tank linings, , COMMON POLYMERS- MONOMERS AND USES., , 1, , 2, , Polymer, Polythene, , Monomer, Ethene, , PVC, (Polyvinyl, chloride), , Vinyl chloride, , Uses, 1. As packing material., 2. In the manufacture of bottles,, toys etc., 3. As insulation for electric wires, 1., 2., 3., 4., , 8, , For making pipes, For roofing and flooring, For making rain coats and bags, For cable insulation, , Chemistry Notes, GPC Cherthala
Page 9 :
3, , Nylon 6,6, , Hexamethylene diamine and, Adipic acid, , 1. Fabrics, Ropes and nets, 2. Threads in the bristles for tooth, brushes, 3. Carpets, 4. Parachutes, 5. Water proof swim suites, , 4, , Bakelite., , Phenol and Formaldehyde, , 1., 2., 3., 4., 5., , HCHO, Formaldehyde, , 9, , For making electrical switches, Handles of utensils, combs, Jewelry, Radio cabinets, , Chemistry Notes, GPC Cherthala