Page 1 :
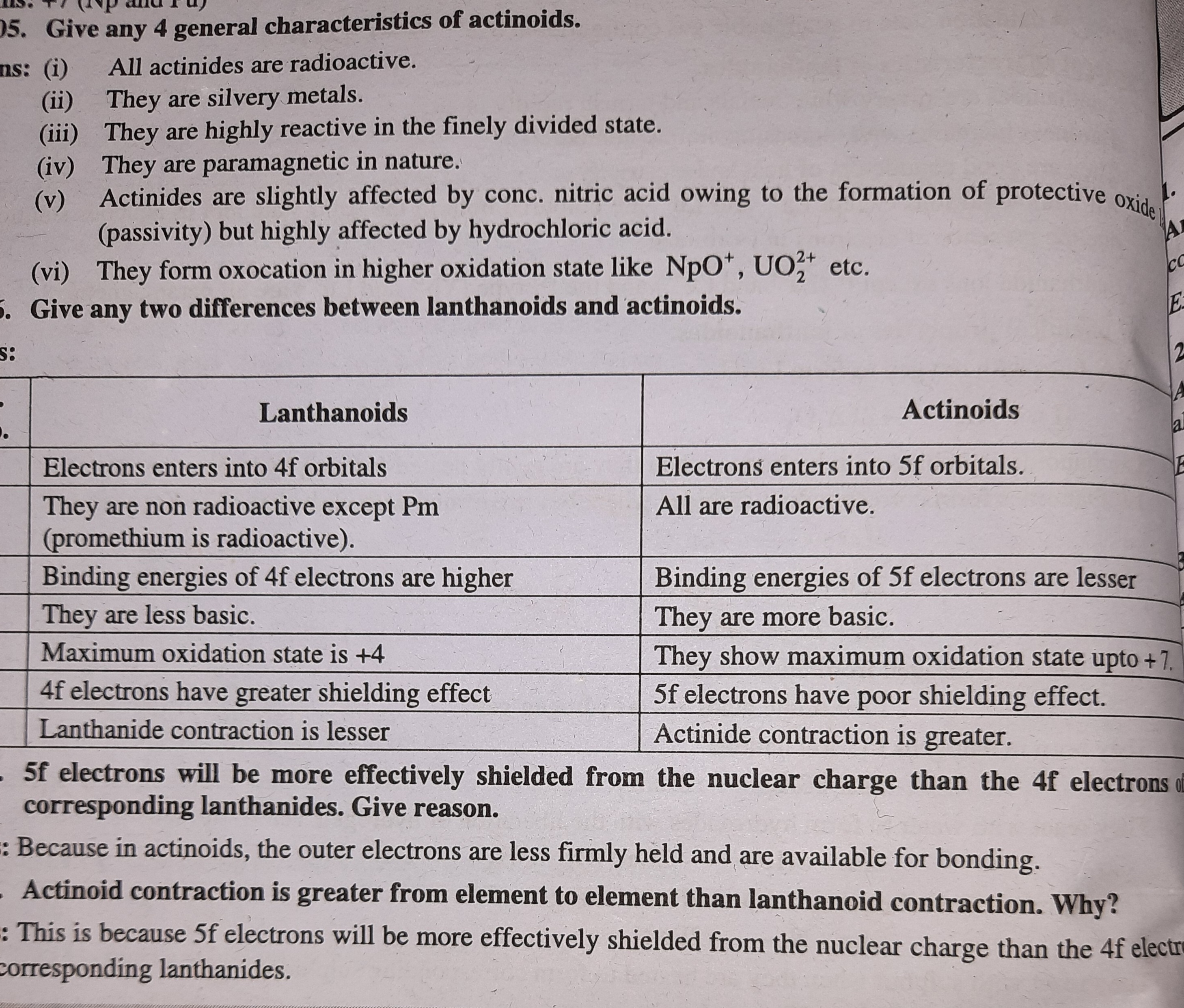
emee TF VAN owe, , )S. Give any 4 general characteristics of actinoids., ns: (i) All actinides are radioactive., , (ii) They are silvery metals., (iii) They are highly reactive in the finely divided state., , (iv) They are paramagnetic in nature., Actinides are slightly affected by conc. nitric acid owing to the formation of protective Ox; te ;, , (v), (passivity) but highly affected by hydrochloric acid. |, _ (vi) They form oxocation in higher oxidation state like NpO*, UO} etc., . Give any two differences between lanthanoids and actinoids., , Lanthanoids |, Electrons enters into 4f orbitals Electrons enters into 5f orbitals. a, All are radioactive., , , , (, , gia, So, , , , 5:, . Actinoids, 5, , They are non radioactive except Pm, , (promethium is radioactive)., Binding energies of 4felectrons arehigher __| Binding energies of 5f electrons are lesser), They show maximum oxidation state upto +1, |, , 4f electrons have greater shielding effect 5f electrons have poor shielding effect., Lanthanide contraction is lesser Actinide contraction is greater., , , 3f electrons will be more effectively shielded from the nuclear charge than the 4f electrons 4d, corresponding lanthanides. Give reason. :, , : Because in actinoids, the outer electrons are less firmly held and are available for bonding., , , , , Actinoid contraction is greater from element to element than lanthanoid contraction. Why?, , : This is because 5f electrons will be more effectively shielded from the nuclear charge than the 4f elect, -orresponding lanthanides. : 3 |