Page 1 :
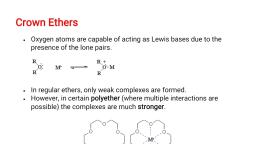
e For example, 14-crown-4, with the smallest cavity has the highest, affinity for Li*, 15-crown-5 for sodium cation, whereas 18-crown-6, forms the strongest complexes with K’., , e The oxygen atoms are well situated to coordinate with a cation, located at the interior of the ring, whereas the exterior of the ring is, hydrocarbon like, hydrophobic., , e The relationship between a crown ether and the ion it binds is also, known as host-guest relationship., , These compounds are important co-solvents., , e Itis possible to dissolve ionic compounds in organic solvents, using crown ethers., , « It allows ionic systems such as KF,KCN and potassium acetate to, be dissolved in organic solvents and used as reagents where the, metal ion is in a complex, but the anion is unsolvated or naked and, therefore quite reactive., , e Effect of a Crown Ether on the Solubility of KMn04 in Benzene., Normally KMn0Oz is intensely purple and is completely insoluble in, benzene which has a relatively low dielectric constant. In the, presence of a small amount of crown ether, KMn04 dissolves in, benzene (the reddish purple colour caused by the permanganate, ions in solution)., , e For this reason crown ethers are useful in phase transfer catalysis, and are called phase transfer catalysts., , _Cryptands, , (a) The potassium complex of the crown ether 18-crown-6. Note how the cation is nestled within the, central cavity of the molecule and interacts with lone pairs of electrons on the oxygen atoms., , (b) The potassium complex of 2, 2, 2-cryptand, showing how the cation is almost hidden by the cryptand., Cryptands solvate cations via lone pairs of electrons on both oxygen and nitrogen atoms., , e Cryptands are more nearly spherical analogues of crown ethers and, are even more powerful and selective complexing agents., , e Cryptands (from the Greek kryptds, meaning “hidden”) are, compounds that can completely surround a cation with lone pairs of, electrons on oxygen and nitrogen atoms., , (a) Crown ether (b) Cryptand