Page 1 :
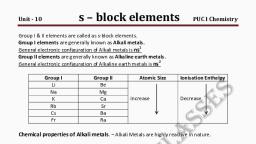
Chemical Properties of Alkali Metals, , The alkali metals are highly reactive due to their large size and low, ionization enthalpy. The reactivity of these metals increases down the, group., , (i) Reactivity towards air, , » The alkali metals tarnish in dry air due to the formation of their, oxides which in turn react with moisture to form hydroxides., They burn with oxygen to form oxides., , >» Oxygen has a different oxidation state in them. Smaller Lithium, forms a normal oxide, while sodium forms peroxides and the larger, atoms (K, Rb, Cs) form superoxides., , > The superoxide 0° ion is stable only in the presence of large, cations such as K, Rb, Cs., , ALi + O2 -> 2Li20 (Oxide; Oxidation Number of Oxygen= -2), 2Na + O2 ~> 2Na202 (peroxide; oxidation Number of Oxygen=-1), , M + O2 -> MOz2 (superoxide; Oxidation Number of Oxygen= -1/2), (M=K, Rb, Cs), , » In all these oxides the oxidation state of the alkali metal is +1., >» Metal and their oxides react with water to ultimately yield hydroxides., , LiiO + H20 = 2LIOH, Na2O2 + 2H20 = 2NaOH + H202, , 2KO2 + 2H20 += 2KOH + H202 + O2, , » Lithium shows exceptional behaviour in reacting directly with, nitrogen of air to form the nitride, LisN as well., , > Since the alkali metals react with nitrogen, oxygen and water in the, air, alkali metals are normally kept in kerosene oil., , (ii) Reactivity towards water:, » Alkali metals react with water to form basic hydroxides and, liberate hydrogen., , 2M + 2H20 = 2MOH + He + AH, (M=Alkali Metals), > The reaction of the metal is exothermic and the enthalpy, increases from Li to Cs. Alkali metal floats on the water during the, reaction. The density of Na and K are lower than water. In heavier, , alkali metal, reaction enthalpy is high such that the metal gets, melted and rises to the surface., , >» Hence, the reaction with water becomes faster, highly exothermic,, and explosive leading to fire from Li to Cs.