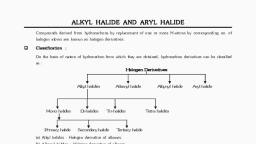
Page 1 :
GENERAL ORGANIC CHEMISTRY, , , Reaction : Breaking of old bond and formation of new bond is known as chemical reaction, , A B + X Y, , A, , X + B Y, New bonds, , Old bonds, , A sequential account of each step, describing details of electron movement, energetics during bond cleavage, and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as, reaction mechanism., Species on which reagent is attacking is known as substrate or reactant., Species which attack on substrate, is known as reagent., , , Type of cleavage of bond :, (A), , Heteroly t ical, , (B), , Homolytical, , cleavage/fission., cleavage/fission., , , , C, , + ×, , C ×, , +, , |V Ionic cleavage, or, |, Heterolytic, fission, ZW, , C, , + ×, , Z, , Z, , , C .× Z, , Un Ionic cleavage, or, Homolytic fission, , Reaction, intermediate, (A), , Cleavage in which unequal distribution of electrons takes place during the bond cleavage is known as, heterolytical cleavage. Due to unequal distribution of electrons, ions are formed. That’s why it is also, known as ionic cleavage or heterolytical cleavage., , , C, , + ×, , Z, , C ×Z, , C, , (B), , , Z, , × +, , Cleavage in which equal distribution of e–s takes place during the chemical reaction is known as homolytical, cleavage., , C, , , ×, , Z, , C, , + ×, , Z, , Due to equally distribution of electrons, without charge unpaired electrons species is formed, which is, known as free radical and cleavage is known as unionic cleavage/homolytical fission., , , , By both cleavage [ionic/non ionic] three type of species are formed [One carrying positive charge, other, carrying negative charge and third one is neutral with unpaired electrons] is known as reaction intermidiate.
Page 2 :
AT TACKING RE AGENTS, The species which attack on a substrate molecule or intermediate and form a product is called as attacking, reagent. These are of two types :, (A), , Electroph i lic reagent or electroph i le s:, Electrophilic, , (electro, , +, , philic), , (electron +, , loving), , The reagent which attacks on the negative of the molecule or loves electrons are called electrophiles., Electrophiles may be positively charged or electron deficient molecule (molecule with sextet or septet)., (i), , Posit ively charged electroph i le s :, , , , , H , S O3 H, , , , NO , N O2, , ,, , , ,, , X, , , , , ,, , R , C6 H5 N2, , , ,, , ,, OH CH3, , C H2, , , , C, CH2, , , , CH, , O, (ii), , Neutral electrophi le s :- Which possess a electron dificiency., , (a), , All Lewis acids as :, BF 3, AlCl 3 , SO 3, ZnCl 2, BeCl 2, FeCl 3 , SnCl 2 , CO 2, SnCl 4 ., , (b), , The neutral atom that accept electrons from the substrates :, , *N, *, *OCl, R – * – X, * – Cl, CH – C, * = O, R C, * , R–OH, > C, Mg, R–Cl , R–O, I, 3, H, , O, R–C, •, , O, O , R–C, •, , •, , R–C, O, , O, O, •, •, , R–C–NH2 , R–C–OH, , R–C=C=O, R–N=C=O, •, •, O–R, H, , The star (*) indicates the atom that accept electrons., (c), , Free radicals, carbenes & nitrene acts as electrophiles., , (d), , Reasent with electrophilie centre :, •, , •, , •, , •, , •, , •, , •, , H , (H–F, H–Cl, H–Br, H–I), H–O–Cl , H–O–Br , O=N–Cl , O=N–Br , Br–Cl , I–Br, (B), , Nucleoph i lic reagent or nucleoph i le s :, Which attack on the positive site of the substrate or loves nucleus., Nucleophilic, , (Nucleo + philes), , (Nucleus + loving), , Nucleophiles may be negatively charged ions or possess a lone pair of electron or donate an unshared electron, pairs., (i), , Negatively charged nucleophiles., , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , H, OH, OR, CN, X( I, Br, Cl, F), R, R– COO, NH2 , CH2 –COR, SH, HSO4 , NO3 , RS , CO3 ,, , , , , , , , , CH 2= CH, N=N=N., (ii), , All Lewis base which contains lone pairs :, , , , , , , , , , , , , , , , , , , R , N H3 , R N H2 , R3 N , R S, H, RS, R , H2 S, ,R O, H2 O, H ,R O, , , , , ,
Page 3 :
*, *, * , CH – CN, *, R, * , NaBH, * CuLi , R, * Zn, R, * – Li, LiAlH, * Cd, Bu, * AlH, R, – Mg – X, R, 2, 2, 2, 2, 4, 4, 3, , (iii), , The star (*) indicates the atom which donates electrons to the substrate., Ambident nucleophile : Nucleophiles which have two sites of electron rich centre or in which two or, more atoms bear an unshared pair of electrons., , , Examples :-, , K—O—N, , O,, , NH 2 —OH,, , , , ••, , K CN, , , , CH2–C–R, , CH2=C–R, , O, , O, , , Resonating structures are also abmbident nucleophile., Amphiphile : Molecule containing a multiple bond between carbon and a more electronegative atom can, act both as electrophiles or nucleophiles., For example :, , CH3 C N, , >C O, electrophilic, S .N o ., , , , nucleophilic, , electrophilic, , nucleophilic, , E l ec t r o ph il e, , Nu c l eo ph il e, , 1, , Accepts the electron pair, , Supplies the electron pair, , 2, , Electron deficient, , Electron rich, , 3, , Attacks the points of high electron density, , Attacks the point of low electron density, , 4, , Lewis acid, , Lewis base, , 5, , Possess an empty orbital to receive the, electron pair, , Possess an electron pair which is loosely held and, can be supplied easily, , 6, , Usually positively charged species, , Usually negatively charged species, , 7, , Forms an extra bond with the nucleophile, , Increases its covalency by one unit, , ELECTRONIC EFFECTS :, There are four effect which affect the chemical reaction are –, (1) Inductive effect, , , , (2) Mesomeric effect, , (3) Hyper conjugation, , (4) Electromeric effect, , INDUCTIVE EFFECT :, , , Polarity developed in Carbon–chain due to the shifting of bond electron by the group or atom, present on carbon-chain is known as inductive effect,., , , , Discovered by scientist Ingold., , , , It is effective upto 3 or 4 carbons. After 3 or 4 carbons it becomes neutralized., , 1, d is t a n ce, Inductive effect is measure with respect to hydrogen atom that means inductive effect of hydrogen is, always zero., So Magnitude of, , , , I effect∝, , , , It is a permanent effect., , , , Some atoms or groups have a greater tendency to attract the shared electron of the covalent bond. Such, atoms or groups acquire partial negative charge by receiving electron density from the covalent bonds, of the chain. Therefore, these are classified as the groups exerting negative inductive (– I) effect.
Page 4 :
, , If shifting of electron takes place from carbon chain to group or away from the carbon chain or towards, the group , then it is known as (–) I effects., , , Z, , , C– C, C, C, 3, , 2, , 1, , (I), , C1() > C2 () > C3 (), , , Due to –I effect positive charge develops over carbon chain or carbon chain becomes electron deficient, such type of carbon chain will be more reactive towards nucleophile or less reactive will be towards, electrophile., Group which shows (–) I are The decreasing order of negative inductive effect of some important atoms and groups is given below :, , , , , OR2 > –NR3 >–NH3 > –NO2 > –SO3H > –CN >, , Order of – I effect, , –COOH > –X > –OH > –OR > –NH2 > –C6H5 > –H, , , Some groups are electron donor and therefore acquire partial positive charge by increasing electron, density in the covalent bonds of a chain. Such groups exert positive i nductive (+ I) effect., , , , Or if shifting of electron takes place from group to carbon chain or away from the group or towards, the carbon chain then it known as (+) I effect, , , C– C, 3, , , C, 2, , , C Z, 1, , (II), , C1() > C2 () > C3 (), , , Due to +I effect electron density increase on carbon chain or negative charge comes on carbon chain, such type of carbon chain will be more reactive towards electrophile or will be less reactive towards, nucleophile., Group which shows +I effect are., O, , , , , –O–O–C , all alkyl groups, , , In alkyl groups megnitude of +I effect size of alkyl group, – CH 3 < – CH 2CH 3 < –CH 2CH 2CH 3, , < –CH 2 CH 2 CH 2CH 3, , In isomeric alkyl group megnitude of +I effect number of, , branche s., , CH3, –CH2CH2CH2CH3 < –CH2–CH–CH3 < –C–CH3, CH3, , , APPLICATION OF I-EFFECT, , (1), , Stability of carbocation :, , CH3, , (a), , If number of + I groups increases then stability of carbocation increases., , (b), , If number of –I groups decreases then stability of carbocation increases.
Page 6 :
Aci di c a nd basi c strengt h :, , , Acidic strengt h :, +, , H donar, Acid, e– acceptor, (i), , If No. of – I groups increases then acidic strength increases., , (ii), , If No. of + I groups increases then acidic strength decreases., , a cid ic s t r e n g t h No . o f - I g r o u p s , , 1, No . o f + I g r o u p s, , Example :, (i), , CH3 C, O, , OH, , Cl, , CH2 C, O, , , , –H, , , , –H , , , , CH3, , (ii), , C, , OH, , O, , Cl, , CH2 C, , O, , O, O, +I of –CH3, –I of –Cl, so anion is more stable, so anion is less stable, and corresponding acid is more acidic., CH3 CH2 CH COOH > CH3 CH CH2 COOH > CH2 CH2, , F, , F, , F, , minimum distance of F, from –COOH maximum –I, of F. So maximum acidic., , , Basic strengt h :, –, , OH donar, Base, , e– donar, +, , H acceptor, (i), , If No. of + I groups increases then basic strength increases., , (ii), , If No. of – I groups increases then basic strength decreases., , B a s ic s t r e n g t h No . o f + I g r o u p s , , 1, No . o f – I g r o u p s, , Example :, , CH3, CH3 C OH, , >, , CH3, Maximum +I., , CH3 CH, , OH, , CH3, , Maximum tendency to donate l.p., Maximum basic., , >, , CH3 CH2 OH, , CH2, , COOH
Page 7 :
Ex., , Explain— Basicity order in aqeous solution and in liquid phase., , Et 2 NH > Et 3 N > Et NH 2, , S o l . Due to steric hindrance in 3° amine, it is less basic, than 2° amine., Steric hindrance of three –C 2H 5 group protect the lone pair of nitrogen from the attack of H ., , H, H H, HH C, H, C, C H, .., H, H, N, H, , C, , H C H, H C H, H, , , , , , , , , But i n gaseous phase basic order is R 3 N >R 2 N H > R N H 2 > N H 3, Some other basic order of different amine if alkyl group would be change (in aq. medium), , Ex., , Alkyl groups (R–), , Relative base strength, , (i), , CH 3 –, , R 2 NH > RNH 2 > R 3 N > NH 3, , (ii), , C 2H 5 –, , R 2 NH > R 3 N > RNH 2 > NH 3, , Ethyl amine is more basic then aniline, why ?, , S o l . Due to the + I effect of ethyl group., Ex., , Cl–NH 2 is less basic then methyl amine, why ?, , S o l . Due to – I effect of –Cl group and p–d conjugation., Ex., , Correct basic order of the following different amine in chlorobenzene medium is NH2, , (A) CH 3 – CH 2 – NH 2, , (B), , (C), , (D), , N, CH3, , N, H, , S o l . (C > D > B > A), Ex., , Which is most basic among the following :, (A) CH 3 NH 2, , (B) CH 3 CH 2 NH 2, , (C) NH 3, , (D) (CH 3) 2 CHNH 2, , S o l . (D), Ex., , Which is most acidic compound –, , OH, , OH, , OH, , OH, , NO2, (A), , (B), , (C), , NO2, S o l . (B), , NO2, , (D)
Page 8 :
, , MESOMERIC EFFECT OR RESONANCE EFFECT :, , , , Polarity developed in conjugate system by the complete transfer of non–bonding electron or –bond electron, due to the group or atom attach with conjugate system is known as mesomeric effect., , , , If transfer of pi-bond electron takes place from conjugate system to group then it is known as negative, mesomeric (–M) effect., , , O, , CH2 =CH — N, O, , , , , O, , CH2 —CH = N, , , O, For –M effect, group should have either be positive charge or should have vacant orbital., , , , Due to –M effect positive charge comes over conjugate system or due to –M effect electron density decrease, in conjugate system, such type of conjugate system will be more reactive towards nucleophile or will be less, reactive towards electrophile., , , , Group wh ich shows –M effect are –NO 2 , –CN, –SO 3H , – CHO, –COR, –COOH, –COOR , –COX, –CONH 2 etc., , , , If transfer of non bonding electron takes place from group to conjugate system then it is known as positive, mesomeric (+M) effect., , .., CH2 =CH – CH =CH – CH =CH – NH2, , , CH2 = CH – CH =CH – CH — CH =NH2, , , CH2 = CH – CH – CH = CH – CH = NH2, , , CH2 – CH = CH – CH =CH – CH = NH2, , , For +M effect, group should have either be lone pair of electron or should have negative charge., , , , Due to + M effect negative charge comes over conjugate system or electron density increase on conjugate, system such type of conjugate system will be more reactive towards electrophile or will be less reactive towards, nucleophile., , , , Group wh ich shows + M effect are -, , – O , –NH, –NR2 , –NHR, –NH2 , – OH, –OR, –SH – SR, – F, – Cl, – NHCOR , –O–COR etc., , , In mesomeric effect polarity or charge migrate from one end to another end. During charge transfer, energy, releases from the conjugate system which increase the stability of conjugate system also., , , , Due to charge transfer compound form more then one structure. without change in atomic orientation these, structures are known as resonating structures.
Page 9 :
, , These structures are helpful in explanation of chemical reactivity or the chemical reaction of the compound, thats why we can say resonance phenomenon is the result of mesomeric effect or delocalisation., (i), , Resonating structure are not the real structures of conjugated compounds., , (ii), , The real structure of conjugated compound is a hybrid of all resonating structures. This phenomenon is, known as resonance, mesomerism or delocalisation., , (iii), , Thus resonance is nothing but hybridisation of resonating structures and resonance phenomenon will, take place in conjugated compounds., , (iv), , Conditions of Resonating Structures : Resonance structures should fulfil following conditions :, , (a), , All resonating structures must have the same arrangement of atomic nuclei. Resonance differs from, tautomerism in this very important aspect., O, | , R – C = O –H, , : :, , O, ||, R – C – O –H, , Positions of atomic nuclei in (I) and (II) are same., 3, , 3, , O, OH, ||, |, CH3 – C – CH3 CH3 – C = CH2, 1, , 1, , (I), , (II), , Position of hydrogen nuclei in (I) and (II) are different, hence (I) and (II) are not resonating structures, they, are tautomer., (b), , The resonating structures must have the same numbers of paired and unpaired electrons. However, they, differ in the way of distribution of electrons., , ••, , •, , ••, , •, O=N—O, •, ••, ••, , , Total number of, paired electrons = 16, unparied electron =1, , ••, •, •, ••, , •, , ••, , O—N=O, ••, , , Total number of, paired electrons = 16, unparied electron =1, , , , The energy of the different resonating structures must be the same or nearly the same., , , , All atoms that are part of the delocalisation system must be in a plane or be nearly planar., (v), , All atoms of the resonating structure should follow the octet rule., For Example: All atoms follow octet rule., , , NH3, , NH3, , , I, , Nitrogen does not follow its octet rule, hence (II) is not resonating structure (I), , II, , , , Condit i ons for re sona nce :, , 1., , If there are two bonds at alternate position then e– of one bond are transferred towards another bond., (According to I–effect).
Page 10 :
Example :, , , CH2 CH CH CH2 CH2 CH CH CH2 CH2 CH CH CH2, , (ii), , CH2 CH C H CH2 CH C H, , , , , , O, (iii), 2., , , , , , , , (i), , O, , CH3 CH CH CH CH2 CH3, , , , , , CH CH CH CH2, , If there is one lone pair or a negative charge and one bond are at alternate position then e– of lone pair, or negative charge are transferred towards bond., Example :, , CH2, , (ii), , , CH2 CH CH2 CH2 CH CH2, , (iii), , , .., , CH2 CH O CH3 CH2 CH O CH3, , (iv), 3., , .., , , CH OH CH2 CH OH, , (i), , , , , , CH2, , , CH O CH2 CH O, , If there is one positive charge and one bond are at alternate position then e – of bond are transferred, towards positive charge., , , Example :, Note :, , , , CH2, , transferred, 4., , , , CH2 CH CH2 CH2 CH CH2, , C H, , does not show resonance because due to more EN of oxygen e – are always, towards oxygen., , O, , If there is one free e – and one bond are at alternate position., Example :, , 5., , CH2, , (ii), , ., ., CH3 CH CH CH2 CH3 CH CH CH2, , CH2, , If there is one lone pair or negative charge and one positive charge are at adjacent atoms then e – of lone, pair or negative charge are transferred towards positive charge., Example :, , , , ., ., CH CH2 CH2 CH, , (i), , , , .., CH2 OH CH2 OH, , Characteristics of Resonance :, (i), , In resonance only e – are transferred not atoms., , (ii), , The number of e – or number of unpaired or paired e – in all resonating structures should be same., , (iii), , The energy of resonating structures is almost same., , (iv), , It is permanent effect., , (v), , All the resonating or canonical structures must confirms to Lewis structures.
Page 11 :
, , Draw re sonat i ng str ucture s :, .., OH, , 1., , , , , , OH, , , , OH, , , , , , , , , , , , OH, , , , , , .., OH, , 5-Resonating structure, , , CH2, , CH2 , , 2., , , , , , CH2, , , , , , , CH2, , , , , , CH2, , 5-Resonating structure, , , CH2, , 3., , CH2, , , , , , , , , , CH2, , , , CH2, , , , , , , , CH2, , 5-Resonating structure, , C H, 4., , C H, , , , O, , , , , , , , O, , , , C H, , , , , , , , O, , C H, , C H, , O, , O, , , , 5-Resonating structure, , ., . CH2, ., , , 5., , CH2, . . ., , , , . ., ., , ., , CH2, , , ., CH2, , . .CH2, , , 5-Resonating structure, , , , , 6., , , , .., , , , , , N, H, , , , , , , , , , , , , , , , N, , N, , N, , N, , H, , H, , H, , H, , .., , N, H, , 6-Resonating structure, , .., NH2, 7., , , , , , , , , , , , .., NH2, , , , NH2, , NH2, , NH2, , , , , , , , , , 5-Resonating structure, , , Hybrid re sonat i ng str ucture :, , CH2 CH CH CH2, 1, , 2, , 3, , I, , 4, , , , , , CH2 CH CH CH2 , 1, , 2, , II, , 3, , 4, , , , , , CH2 CH CH CH2, 1, , 2, , 3, , 4, , III, , In the structure I C1—C 2 has double bond while in II and III C1—C 2 has single bond, so C 1—C 2 shows bond, length in between single and double bond similarly in structure I C 2—C 3 has single bond. While in structure, II and III C 2 —C 3 has double bond, so C 2—C 3 shows bond length in between single and double bond.
Page 12 :
So hybrid resonating structure from all its resonating structures is :, CH 2, , CH, , CH, , CH 2, , Hybrid structure, , Example :, (i), , CH2, , .., CH OH, , , , , , CH2 CH OH, , I, , CH2, , CH2 CH CH2, 2, , (Hybrid structure), , , 3, , , , , , CH2 CH CH2, 1, , 2, , CH2, , , , CH, , CH2, , 3, , (Resonating structure), , , CH, , , , , , 1, , , , OH, , II, (Resonating structure), , (ii), , , , , , , , , , (Hybrid structure), , Re sona nce energy :, The difference in the experimental and calculated energies (heat of hydrogenation) by which the compound, is stable, is known as the resonance or delocalization energy. Higher the value of resonance energy, greater, is the resonance stabilization., , , , Re sona nce energy (R.E.) of Benzene :, , , The Resonance energy of benzene is calculated from the heat of hydrogenation as given below :, H2 , , +, , +, , , , + 3H2, , 28.6 Kcal., , + 3 × 28.6 Kcal. (=85.8), , but experimental value is 49.8 Kcal. so,, Resonance energy = Calculated value – Experimental value, = 85.8 – 49.8 = 36 Kcal., , , APPLICATION OF M- EFFECT OR RESONANCE EFFECT :, , , , Stability of carbocation :, (a), , Stability of carbocation is increased by resonance., , (b), , Aromatic compound are more stable than non aromatic compound., , Example : Compare stability order of :(i), , , , CH2 CH CH2, , , , >, , stable by resonance, , +I of Alkyl group, , , , 1°, (iii), , , , CH2 <, , (ii), , >, , CH3 CH2 CH2, , 2°, , –I of Alkenyl group, , , <, , CH3, , 3°, , , , , , , , CH2, , CH2, , CH2, , , , , Re sonance increases, stability increases, , , , CH2 CH CH CH2
Page 13 :
, , (iv), , , , ,, , , , ,, , (I), , (II), , stable by resonance, , (III), , more resonance, , localized ve charge, , stability order II > I > III, , , Stability of carbanion :, (a), , Stability of carbanion is increased by resonance., , Example : Compare stability order of :, , , (i), , CH2 CH CH2, , , , , , ,, , CH2 CH ,, , stable by resonance, , CH3 CH2, , – ve charge on more EN, , atom, , stability order I > II > III, , , , , (ii), , ,, , ,, , (I), , (II), , stable by resonance, , more resonance, , stability order II > I., (iii), , , , , , CH2 — N O 2, stable by resonance, , CH2 —CH 2 —NO 2, no resonance, , , , C H 3 — CH — N O 2, stable by resoncance, but +I of CH 3, , stability order I > III > II, , , Stabi lit y of free radicals :, (a), , Stability of free radicals is increased by resonance., , Example : Compare stability order of :, ., ., (i), CH2 CH CH2, CH2 CH, less resonance, , (ii), , ., CH2 CH CH CH CH2, , no resonance, , stability order III > I > II, ., ,, , more resonance, , ., , ., , ,, , resonance, , more resonance, , localized, , stability order II > I > III, Equal re sonat i ng str ucture s : Resonat ing structures in which there is same charge on same atom., , Example :, , , , H C O H C O are equal R.S., , , O, , O, , Unequal re sonating structures : Resonating structures in which there is same charge on different atom, or different charge on same atom or different charge on different atom., , , Example :, , , , CH2 CH O CH2 CH O are unequal R.S., .., , , CH2 OH CH2 OH, , Note : Equal resonating structures are more stable than unequal resonating structures.
Page 14 :
, , Acidic a nd Basic strengt h :, (a), , Acidic strengt h :, , A cidic stre n gth M I , , 1, 1, , M, I, , Ex : Carboxylic acids are more acidic than phenols, why ?, , R, , Sol., , C OH, , OH, , , , , , O, , , , , , , C O, , R, , C O, , , , O, , , , R, , , , –H, , –H, , , , O, , O, , 2, equal R.S. more stable anion, so corresponding acid is more acidic, , O, , , 5, unequal R.S. less stable anion, , Ex : Phenol is more acidic than alcohols why ?, Sol., , R OH, , Ph OH, , , , , , , , –H, , , , –H, , R O, , Ph O, stable by resonance, So, it is more acidic., (b), , no resonance., , Basic strengt h order :If there is more resonance of lone pair or negative charge then it will be more stable, means less basicity., , Basic strength M I , , 1, 1, , M –I, , Ex : Give basic strength order :, (i), , .., NH2, , .., NH CH3, , l.P. is stabilized by, , no resonance of l.p., , l.p. is stabilized by resonance, , resonance, , so maximum basic, , stabilized and +I of CH 3, , basic order —, , (ii), , .., CH2 NH2, , II > III > I, , NH2, , .., N, , .., N, H, , stable by, , localized, , resonance, , l.p. on more EN, , basic order —, , III > II > I
Page 15 :
Ex : Give basic strength order for :, .., NH2, , .., NH, , (i), , CH2, , .., NH2, , diphenyl amine, , benzyl amine, , more resonance, , no resonance, , Sol. 3 > 2 > 1, Ex : Aniline is less basic than alkyl amine, why ?, S o l . Due to delocalization of l.p. of nitrogen in aniline, aniline is less basic., Ex : Which is weakest base :, (i) C 6 H 5 —CH 2 —NH 2, , (ii) C 6 H 5 —CH 2 —NH—CH 3 (iii) O 2 N—CH 2 —NH 2 (iv) CH 3 —NH—CHO, , S o l . (iv) due to resonance of l.p., 7., , Reactivity of benzene : Characteristic reaction of benzene is electrophilic substitution (ESR), , , , , E, , , , -H, , , H, E, , , , (product), E, , In benzene ring due to more electron density first attack will be of electrophile., 8., , Rule s for stabi lit y of re sonat i ng str ucture (R.S) :, (i), , Non-polar R.S. is more stable than polar resonating structures., , (ii), , In polar R.S. complete octet is more stable than incomplete octet., , (iii), , For incomplete R.S. positive charge on more EN is less stable., , Example : Arrange the following for stability order., (i), , , , , , , , , , CH2 CH Cl, , CH2 CH Cl, , CH2 CH Cl, , non-polar, , complete octet, , incomplete octet, , stability order : 1 > 2 > 3, , , , , (ii), , , , R C OH, , R C O H, , non-polar, , O, , complete octet, , , , <, , R C O, incomplete octet, , R C, , , , O, , complete octet, , (Acylium ion), (iv), , , , CH2 CH O, , >, , R C OH, , , , , O, , O, , (iii), , , , , , CH2 CH O, , negative charge on, , negative charge on, , more EN, , less EN, , incomplete octet
Page 17 :
Ex., , Give acidic strength order for, (i), , OH, , OH, , OH, , OH, , CH3, CH3, CH3, , , , , , , –H, , –H, , , , O, , O, , , , –H, , , , , , O, , , , , , , , , , , , –H, , O, , CH3, CH3, more +I and, H-effect of CH3 ,So, anion is minimum stable, Acidic order 4 > 2 > 3 > 1, , NO2, , maximum stable anion, so corresponding acid is, maximum acidic, , OH, , OH, (ii), , CH3, less +I and, H-effect, , H= 0, only +I, , OH, , NO2, NO2, , OH, NO2, , NO2, , NO2, maximum –M and –I, so maximum acidic, Acidic order 1 > 2 > 3 > 4, Ex : Give basic strength order for :, , NH2, , NH2, , NH2, , NH2, , NO2, , Cl, , CH3, , OCH3, , –M, , –I, , +I, , +M, , (i), , So basic strength order IV > III > II > I, Ex : What is the increasing order of resonance stabilization of benzene, naphthalene and anthracene., S o l . The values of resonance energy for benzene, naphthalene and anthracene are 36, 76 and 85 Kcal per mole, respectively. It is clear from these values that the resonance stabilisation of naphthalene is more than that of, benzene and less than that of anthracene. Thus, the increasing order of resonance stabilisation is as follows., , Benzene, , Naphthalene, , Anthracene
Page 18 :
Phenol : 9.98/1.10 × 10 –1 0 pK a /K a value, Subtituent, , O–, , M–, , P–, , (i), , Me, , 10.28/0.63 × 10 –10, , 10.08/0.98 × 10 –10, , 10.14/0.67 × 10 –10, , (ii), , NO 2, , 7.23/600 × 10 –10, , 8.40/50 × 10 –10, , 7.15/690 × 10 –10, , (iii), , F, , 8.81/15 × 10 –10, , 9.28/5.2 × 10 –10, , 9.98/1.1 × 10 –10, , (iv), , Cl, , 8.48/77 × 10 –10, , 9.02/16 × 10 –10, , 9.38/6.3 × 10 –10, , (v), , CH 3 –O, , 9.98/1.05 × 10 –10, , 9.65/2.24 × 10 –10, , 9.38/0.62 × 10 –10, , (vi), , OH, , 9.48, , 9.44/3 × 10 –10, , 9.96, , (vii), , NH 2, , 9.71/2.0 × 10 –10, , 8.16/68 × 10 –10, , 10.30, , (viii), , Br, , 41 × 10 –10, , 14 × 10 –10, , 5.6 × 10 –10, , (ix), , I, , 34 × 10 –10, , 13 × 10 –10, , 6.0 × 10 –10, , (x) 2.4- dinitrophenol, , 4.00, , (xi) 2,4,6-trinitro phenol, , pKa = 0.71, , OH, Me, , Me, , 10.60, , OH, Me, , Me, 7.22, , NO2, , OH, 10.17, , Me, , Me, , OH, , Me, , Me, NO2, , 8.25, , 10.50 × 10 –2
Page 19 :
pK a /Benzoic acid 4.20, Substituent, , pKa–, , O–, , M–, , P–, , H, , 4.20, , 4.20, , 4.20, , 4.20, , Me, , –, , 3.91, , 4.27, , 4.37, , NO 2, , –, , 2.17, , 3.48, , 3.43, , F, , –, , 3.27, , 3.87, , 4.14, , Cl, , –, , 2.94, , 3.83, , 3.98, , Br, , –, , 2.85, , 3.81, , 4.00, , OMe, , –, , 4.09, , 4.09, , 4.47, , OH, , –, , 2.98, , 4.08, , 4.58, , NH 2, , –, , 4.92, , 4.72, , 4.98, , –COOH, , –, , 2.98, , 3.46, , 3.56, , H–COOH, , 3.77, , CH 3 –COOH, , 4.76, , CH 3 –CH 2 –COOH, , 4.88, , HOOC–COOH, , 1.23, , HOOC–CH 2 –COOH, , 2.83, , HOOC–(CH 2 ) 2 –COOH, , 4.19, , Maleic acid, , 1.92 (pKa 1 ), , 6.23 (pKa 2 ), , Fumaric acid, , 3.02 (pKa 1 ), , 4.38 (pKa 2 ), , CHC–COOH, , 1.84, , CH 2 =CH–COOH, , 4.25, , F–CH 2 –COOH, , 2.57, , Cl–CH 2 –COOH, , 2.86, , Br–CH 2 –COOH, , 2.90, , I–CH 2 –COOH, , 3.16, , O 2 N–CH 2 –COOH, , 1.68, , Me 3 N + –CH 2 –COOH, , 1.83, , NC–CH 2 –COOH, , 2.47, , EtOCOCH 2 COOH, , 3.35, , MeO–CH 2–COOH, , 3.58
Page 20 :
pK a /K a value-Aniline 4.62, Subtituent, , O–, , M–, , P–, , Me–, , 4.39, , 4.69, , 5.12, , Me–O, , 4.49, , 4.20, , 5.29, , NH 2, , 4.47, , 4.20, , 6.08, , NO 2, , –0.29, , 2.50, , 1.02, , Cl, , 2.64, , 3.34, , 3.91, , Br, , 2.60, , 3.51, , 3.91, , OH, , 4.72, , 4.17, , 5.30, , 4.85, , O, ••, CH–C–NH2, , ~0.5, , –NH–CH3, , –N, , CH3, CH3, , –NH–C2H5, , C2H5, , Ph–N, , C2H5, , O, C, 5.06, , N–H, C, O, , shown some, acidic nature, , 5.11, , 6.56, , NH=C, , NH2, NH2, , 13.6, , NH, NH2, , 12.4, , Ph 2 NH, , 0.9, , Ph–NH–AC, , 0.4, , CH3–C, , 5.69, , (C 2 H 5 ) 2 NH, , 10.93, , Et 3 N, , 10.88, , Et–NH 2, , 10.67, , NH 3, , 9.25, , M e 3N, , 9.80, , M e 2N, , 10.77, , Me–NH 2, , 10.64, , Me, , H3C, HO2C, , NH2, , N, , Me, , CH3, NO2, 4.60, , NO2
Page 21 :
10.50, , N, , Bu 3 N, , 9.87, , Bu 2 NH, , 11.28, , BuNH 2, , 10.61, , NH–CMe3, 7.1, , –0.27, , N, H, , N, H, , N, , 5.23, , N, , 11.22, , N, H, , 2.53, , 4.94, N, , N, 7.03, , N, H, , , 5.2, , N, , HYPERCONJUGATION EFFECT OR H-EFFECT :, Complete transfer of e – of –C–H bond towards bond or positive charge or free electron is called as, H-effect (permanent effect)., , It is also called as non-bonded resonance and given by Nathen and Baker., , , , Condit ions of H-effect :, , 1., , If there is one C–H bond and one positive charge are at alternate position, , H, , , H C CH2, H, , , , H, H C, , H, CH2 , , H, , H, , H C CH2 H, , , , C, , CH2, , , , H, , H, , all are called as hyperconjugation structures or canonical structures., Carbon which is at tached to positively charged carbon is called as –C and H which is at tached to, –C are called as – H. So if number of – H are more then there will be more number of hyperconjugation, structures, so more stable carbocation., 2., , Carbon which is attached to sp2 C is called as –C and H which are attached to -C are called as -H., If there is one –C–H bond and one free e – are at alternate position then there will be H-effect., , ., H, H, H, ., ., , H C CH2 H C CH2 H C CH2 H, ., H, H, H, , H, C, H, , CH2
Page 22 :
3., , If there is one –C–H bond and bond are at alternate position then there will be H-effect., , , H, , CH, H C, 1, 2, , H, , H, , H, , , , , , , , , CH2 H C CH CH2, CH2 H C CH CH2 H C1 CH, 2, 3, 1, 2, 3, 3, 1, , H, , 2, , 3, , , , H, , H, , H, , Special point :, (i), , H3 C, 1, , CH CH2, 2, , 3, , C 1 —C 2 bond length = 1.54 Å, C 2 —C 3 = 1.34 Å., but in propene the actual bond length is 1.54 > b.l. > 1.34 Å, It can be explained by H-effect. So C–C bond length order of, CH 2, , CH—CH, , CH 2, , CH 3 —CH, , CH 2, , CH 3 – CH 3, , is III > II > I, Due to resonance bond length is shortest in I., (ii), , The effectivity order of M, H and I effect, M > H > I, , Note : If there is one –C–H bond and one negative charge at alternate position then there will be no H-effect., , H, , , H C, , CH2, , (No H - effect), , H, no shifting of —C—H bond, because anion have complete octet. (8e – ), , , APPLICATION OF H-EFFECT OR H-EFFECT :, Stability of carbocation :, , , Stability of carbocation can be explained by M, I and H-effect., , , , If more hyperconjugation structures (more -H) then more stable cation., , , , Stability of carbocation No. of canonical structures No. of H., , Ex., , Give stability order for :-, , CH3, (i), , CH3 C, , >, , , , CH3 CH, , CH3, , , , >, , CH3 CH2, , 6 -H, , 3 -H, , Maximum stable, , , CH3, , , , CH3, , CH3, , 9 -H, , (ii), , >, , , , , , >, , >, , CH2, , Zero -H
Page 23 :
, , Stabi lit y of carbon free radicals :, •, , Stability of carbon free radical is explained by M and H-effect., , •, , More hyper conjugation structures (more -H) more stable free radical., , Ex : Give stability order for :-, , CH3, , CH3, ., C, , ., CH3 CH, , ., CH3, , ., CH3 CH2, , CH3, , CH3, , 3°, , 2°, , 1°, , 9 -H, , 6 -H, , 3 -H, , Zero -H, , Maximum stable, Stability order, , , I > II > III > IV, , Stability of alkenes : More hyperconjugation structures (more -H) more stable alkene., (i), , CH 3 —CH, , CH 2 >, , 3 -H, , CH 2, , CH 2, , Zero -H, , more stable, (ii), , Stability order of alkenes will be, , CH3, , C C, , CH3, CH3, H, , C C, , CH3, , CH3, , CH3, , CH3, , CH3, H, , CH3, H, , C C, , C C, , CH3, , CH3, , H, , CH3, , H, , H, , H, , H, , C C, , C C, , H, , CH3, , H, , H, , C C, , H, CH3, , H, H, , Ex : Which of the following alkene is maximum stable., (A) R 2 C, , CR 2, , (B) R—CH, , CR 2, , (C) R—CH, , CH—R, , (D) R—CH, , CH 2, , S o l . (A) due to more substituted alkene., , , Heat of hydrogenat ion :, R—CH, , CH 2 + H 2 R—CH 2 —CH 3 + H (Heat of hydrogenation), , Heat evolved when any unsaturated hydrocarbon are hydrogenated is called heat of hydrogenation (H), If alkene is more reactive towards hydrogen then it will evolve more H., , So,, , Heat of hydrogenation , , 1, 1, , stability of alkene number of H, , Ex : Which of the following has minimum heat of hydrogenation., (i) ethene, S o l . (iv), , (ii) Propene, , (iii) cis-2-butene, , maximum stable alkene means minimum reactive., , (iv) trans-2-butene
Page 24 :
Ex : If Heat of hydrogenation of 1-butene is 30 Kcal then heat of hydrogenation of 1,3-butadiene is ?, (i) 30, S o l . (iii), , (ii) 60, , (iii) 57, , (iv) 25, , 1,3-butadiene requires two moles of hydrogen so heat of hydrogenation should be 60 Kcal but, 1,3-butadiene is stabilized by resonance than propane so heat of hydrogenation of 1,3-butadiene, will not be twice of 30., , Actual H - 60 > H > 30 Kcal., Ex : Which of the following is maximum stable., (i), , Conjugated alkadiene (CH 2, , (ii), , Isolated alkadiene (CH 2, , (iii), , Cumulated alkadiene (CH 2, , (iv), , All are equal., , S o l . (i), , CH—CH, , CH 2 ), , CH—CH 2 —CH, C, , CH 2 ), , CH 2 ), , Due to resonance conjugated alkadiene is maximum stable. Isolated is more stable than cumulated, alkadiene due to H-effect., , , , Reactivity of Benzene :, , H-effect of R groups increases electron density in benzene ring., , , , H, C H, , H, C H, , , , H, , , , H, , due to CH 3 group there is more e – density at ortho and para position so CH 3 is ortho/para directing and, activating group., If H-effect is more than e – density will be more., Ex : Give electrophilic sustitution reaction order :, , Maximum -H., So maximum H-effect, So maximum e – density, So maximum reactive, ESR order I > II > III > IV, , , ELECTROMERIC EFFECT : (E Effect), It is a temporary effect. The organic compounds having a multiple bond (a double or triple bond) show this, effect in the presence of an attacking reagent only. It is defined as the complete transfer of a shared pair of, -electrons to one of the atoms joined by a multiple bond on the demand of an attacking reagent. The effect, is annulled as soon as the attacking reagent is removed from the domain of the reaction. It is represented by, E and the shifting of the electrons is shown by a curved arrow (, ). There are two distinct types of electromeric, effect.
Page 25 :
(i), , Positive Eelctromeric Effect (+ E effect) : In this effect the -electrons of the multiple bond are, transferred to that atom to which the reagent gets attached. For example :, , C = C + H+, (attacking, reagent), (ii), , H, , Negative Electromeric Effect (-E effect) : In this effect the - electrons of the multiple bond are, transferred to that atom to which the attacking reagent does not get attached. For example., , C = O + CN, (attacking, reagent), , , , , C–C, , HCN, , , , CH3 C, , H CH3 C, , O, , O, , H, , C–O, CN, , , , ,, , , , H2O, CH3 C N , CH3 C N, , , , AROM ATICIT Y :, Many cyclic, conjugated compounds possess markedly different physical and chemical properties from those, expected by comparision of their structures with acyclic analogues. The simplest example is benzene, which, may be regarded as the parent compound of the aromatic series. Benzene is a planar, cyclic compound with, a cyclic cloud of delocalized electrons above and below the plane fo the ring, because its -electrons are, delocalized, all the carbon-carbon bonds have the same length-partway between the length of a typical single, and a typical double bond. We also saw that benzene is a particularly stable compound because it has an unusually large resonance energy (36 kcal/mol or 151 kJ/mol). Most compounds with delocalized electrons have, much smaller resonance energies. Compounds such as benzene with unusually large resonance energies are, called aromatic compounds., , (a) Each carbon of benzene has a p orbital., (b) The overlap of the p orbitals forms a cloud of electrons above and below the plane of the benzene ring., (c) the electrostatic potential map for benzene shows that all the carbon-carbon bonds have the same electron, density., For a compound to be classified as aromatic, it must fulfill both of the following criteria., (i), , It must have an uninterrupted cyclic cloud of electrons above and below the plane of the molecule, (often called a cloud)., For the cloud to be cyclic, the molecule must be cyclic., For the cloud to be uninterrupted, every atom in the ring must have a p orbital., For the cloud to form, each p orbital must be able to overlap with the p orbitals on either side of it., Therefore, the molecule must be planar., , (ii), , The cloud must contain an odd number of pairs of electrons.
Page 26 :
Benzene is an aromatic compound because it is cyclic and planar, every carbon in the ring has a p orbital and, the cloud contains three pairs of electrons., The german chemist Erich Huckel was the first to recognize that an aromatic compound must have an odd, number of pairs of electrons. In 1931 he described this requirement by what has come to be known as, Huckel's rule, or the 4n + 2 rule. The rule states that for a planar, cyclic compound to be aromatic, its, uninterrupted cloud must contain (4n + 2) electrons, where n is any whole number. According to Huckel's, rule, then an aromatic compound must have 2(n = 0), 6(n =1), 10 (n =2), 14 (n = 3), 18 (n = 4), etc., , electrons. Because there are two electrons in a pair, Huckel's rule requires that an aromatic compound must, have 1, 3, 5, 7, 9, etc. pairs of electron. Thus, Huckel's rule is just a mathematical way of saying that an, aromatic compound must have an odd number of pairs of electrons., Cyclobutadiene has two pairs of electrons and cyclooctatetraene has four pairs of electrons therefore, these, compounds are not aromatic because they have an even number of pairs of electrons. There is an additional, reason why cyclooctatetraene is not aromatic- it is not planar, it is tub-shaped. We saw that for an eightmembered ring to be planar, it must have bond angles of 135° and we know that sp2 carbons have 120° bond, angle s. Therefore, If cyclooctatetraene were planar, it would have considerable angle strain. Because, cyclobutadiene and cyclooctatetraene are not aromatic, they do not have the unusual stability of aromatic, compounds., , cyclobutadiene, [4]-annulene, , benzene [6], -annulene, , cyclooctatetraene, [8]-annulene, , Monocyclic hydrocarbons with alternating single and double bonds are called an-nulenes. A prefix in brackets, denotes the number of carbons in the ring., Which of the following three-membered ring structures is aromatic ? Cyclopropene is not aromatic because, it does not have an uninterrupted ring of p orbital-bearing atoms. One of its ring atoms is sp3 hybridized and, only sp2 and sp hybridized carbons have p orbitals. Therefore, cyclopropene does not fulfill the first criterion, for aromaticity., , Cyclopropene, , –, , :, , +, Cyclopropenyl cation, , Cyclopropenyl anion, , The cyclopropenyl cation is aromatic because it does have an uninterrupted ring of p orbital-bearing atoms, and the colud contains one pair of delocalized electrons. The cyclopropenyl anion is not aromatic because, its cloud has two (an even number) paris of electrons., +, , +, , +, resonance contributors for the cyclopropenyl cation, , +, , +, , +, resonance hybrid
Page 27 :
Cycloheptatriene is not aromatic. Although it has the correct number of pairs of electrons to be aromatic, (three pairs), it does not have an uninterrupted ring of p orbital-bearing atoms because one of the ring atoms, is sp 3 hybridized. Cyclopentadiene is also not aromaitc. It has an even number of pairs of electrons (two, pairs) and it does not have an uninterrupted ring of p orbital bearing atoms., , cycloheptatriene, , cyclopentadiene, , The criteria for determining whether a monocyclic hydrocarbon compound is aromatic can also be used to, determine whether a polycyclic hydrocarbon compound is aromatic. Naphthalene (five pairs of electrons),, phenanthrene (seven paris of -electrons)and chrysene (nine pairs of electrons) are aromatic., , naphthalene, , phenanthrene, , chrysene, , Pyridine, , pyrrole, , ::, , N, , :, , N, , ::, , :, , In cyclic compounds, when an element other than carbon is present in the ring, they are called heterocyclic, compounds. They are aromatic in nature because of fulfilling both the conditions., , S, , O, , H, Thiophene, , Furan, , In pyrrole, furan and thiophene, the lone pairs are actually in sp3 hybrid orbtials to form the aromatic sextet, (4n + 2) -electrons. In pyridine, the lone pair is in sp2 hybrid orbital, which is not involved in delocalization., Thus, pyridine also has 6 -electrons and is aromatic in nature., , , RE ACTION INTERMEDIATES :, In the study of organic chemistry follwing intermediates are more important :, , , , (i) carbocation, , (ii) carbanion, , (iii) carbon free radical, , (iv) carbene, , (v) Benzyne, , (v) nitrene, , Carbocation : Positively charged 'C' atom as reaction intermediate is called carbocation. Generally it contains, only six electron in three bonds. It is electron deficent species act as electrophilic and lequid acid., , Carbocation, Classical, or, Carbenium ion, , Non classical, or, Carbonium ion, , Note :, , , , , According to modern nomenclature accepted by IUPAC CH3, CHCH, 3, 2 etc. are termed as carbenium ion (but, , , , , not carbonium ion). Althought some authors still refers to CH3, CHCH, n, 3, 2 etc as carbonium ion but in modern, nomenclature it is old pentavalent carbocation (i.e. carbonium ions) are much rare than carbenium ion.
Page 36 :
(ii), , It has 'sp' hybridisation but Linear strucuture., , Z, X, Y, , expected structure (But not real), (iii), , It is ground state of carbene., , (iv), , It is paramagnetic with magnetic moment., µ =, , (3), , n(n 2) =, , 2(2 2) = 2 2 Bohr magnaton., , (v), , Multiplicity M = 2S + 1 = 2 (+1/2 + 1/2) + 1 = 3, , (vi), , It is stronge electrophile., , Generation of carbene :, , , (i), , , CH 2 N N , : CH 2, , diazomethane, (ii), , , CH 2 C O , : CH 2, , R, , R, C= C= O, , (iii), , , , R, , (iv), , By action of base of chloroform, , Cl, Cl, Cl, , (4), , C:, R, , –, , C— H, , OH, –H2O, , Cl, Cl, Cl, , C–, , Cl – C – Cl, , (v), , CCl4 + LiR – R – Cl + LiCCl3, LiCCl3 Cl – C – Cl, , (vi), , LiR, CH2Cl2 , : CHCl, , Reactions of carbene :, (i), , Attachment with nucleophile :, , H, Cl – C – Cl, , H–OH, , O, , O, , Cl – C – Cl, , –HCl, , ••, , H – C – Cl, , ••, , ••, , OH2, , H— C — Cl, , , O– H, , O– H, , O, , O, , , H– C –O, , Cl – C– – Cl, (ii), , Cl – C – Cl, , H–NH2, , +, , H— N — H, H, , H, , , , OH, , H– C–O–H, , H, Cl — C — Cl, N–H, H, , –HCl, , H, NH3, , Cl — C = N, H, , NH3, , H— C N
Page 41 :
(–) 2-Bromo octane (Inversion of configuration)., An SN2 reaction proceeds with complete stereo chemical inversion., SN2 reaction decreases with stearic hindrance and increases by non protic solvent for example DMF and, , DMSO. SN2 reaction decreases in the presence of protic solvent like CH3OH, C2H5OH., , H, H–C–Br, , H, CH3–C–Br, , Br, CH3–C–CH3, , H, , H, , H, , 37, , 1.0, , 2 × 10, , CH3, CH3–C–Br, CH3, , –2, , –4, , 8 × 10, , SN2 reactivity 1° > 2° > 3°, , , , , , , , , Rate – H O R – X [H O .....R ....... X HO – R X ( .g), • As the size of alkyl group increases then chemical reactivity towards SN2 decreases., , , R–Br+Cl, , DMF, , , , RCl + Br, , CH3, , CH3, , Relative rate - CH3CH2Br > CH3CHCH, 2, 2Br > CH3–CH–CH2–Br > CH3–C–CH2–Br, , CH3, 1.0, , , , (2), , 0.69, , 0.33, , –6, , 6 × 10, , Feature s of SN2 React ion :, (i), , Occurs mainly in 1° alkyl halide., , (ii), , Order of reaction is 2, but total step =1, , (iii), , Speed of the reaction increases by non protic solvent ex. DMF and DMSO., , (iv), , Back attack of the nucleophile is there so that stereo chemical inversion occurs., , (v), , Elemental effect so that RI is maximum reactive., , (vi), , No carbocation, so no molecular rearrangement. Only transition state is formed., , SN1 subst itut ion react ion :, , CH3, , , , CH3–C–Br + OH, CH3, Rate =, , CH3, , CH3–C–OH + Br, CH3, , K [RBr], , , , , Slow : R–X R X, , , , , Fast : R OH ROH, Now nucleophilic attack can conceivably take place any time after the heterolysis and thus can involve any, species from the initially formed ion pair to the free carbocation, attack on the free carbocation is random and, yields the racemic modification. The anion clinge side of the carbocation and thus shields this side from, attack ; as result, back side attacks preferred to the extent, that attacks occurs before the ion pair has completely, separated. Inversion of configuration competes with racemization.
Page 42 :
H2O, , , C6H13 C H, CH3, C6H13, HO–C–H, CH3, , H–C–OH, CH3, , (a) Inversion, predominates, , , , C6H13, , (b) Retention, , Feature s of SN1 React ion :, (i), , Rate of reaction = K[RX], Order = one, but occurs in two steps., , (ii), , Carbocation formation takes place so that molecular rearrangement may occur., , (iii), , Racemization takes place., , (iv), , Chemical reactivity 3° > 2° > 1°., , (v), , Elemental effect, so that RI are maximum reactive., , P rop e r t i e s, , SN1, , SN2, , Reacti vity of hali de, , RI > RBr > RCl > RF, , RI > RBr > RCl > RF, , R3CX > R2CHX > RCH2X > CH3X, , CH 3X > RCH2 X > R2 CHX > R 3CX, , Rate is governed by stability of, , Rate is governed by stearic effect, , carbocation that is formed in, , (crowding in transition state)., , leav i ng, , groups, , Effect of str ucture, , ionization step., Effect of solvent, , Effect of nucleoph i le, , Stereochemistr y, , (II), , Rate increases with increasing, , Rate depends on both nature and, , polarity of solvent., , concentration of nucleophile., , Rate of substitution is independent, , Polar aprotic solvents give fastest, , of both concentration and nature, , rates of substitution; solvent of Nu :, , of nucleophile by its dielectric, , is maximum and nucleophilicity is, , constant ., , greatest., , Not stereospecific ; racemization, , Stereospecific; 100 percent inversion, , accompanies inversion when, , of configuration at reaction site., , leaving group is present at a, , Nucleophile attacks carbon from side, , stereogenic centre, , opposite bond to leaving group., , Elimi nat i on react ions :, Elimination reactions, in which two groups are removed from a molecule, neither being replaced by another, group, are the reverse of addition reactions. Usually they involve the loss of two substitutions from vicinal, atoms resulting in the formation of a double or triple bond. Most commonly a proton is lost from one, carbon whereas a nucleophile is lost from the adjacent carbon ; these two carbon atoms are usually to, as -and -carbons, respectively., , X, , , –C–C–, , H, , –HX, , –C=C–, H, , This type of elimination reaction is known -elimination or 1, 2,-elimination reaction.
Page 43 :
, , -Eli mi nati on Reacti on :, In analogy with substitution reaction, -elimination reactions are divide into E 1 (Elimination, unimilecular), and E 2 (Elimination, biomolecular) reactions., E 2 Reacti on : The Biomolecular Mechani sm :, Many elimination reactions are successful only when carried out in the presence of a strong base. The formation, of ethylene on the treatment of ethyl bromide with sodium ethoxide is an example of this type. The rate, of alkene formation is proportional to the concentrations of ethyl bromide as well as that of sodium ethoxide., CH 3 CH 2 Br + C 2 H 5 O CH 2 = CH 2 + C 2 H 5 OH + Br , , , Rate = K [CH 3CH 2Br] [C 2H 5O ], , Two types of mechanism can be written both of which are in agreement with the kinetic data. In the first, mechanism the base abstracts a proton from the -carbon and simultaneously the leaving group departs, from the -carbon along with the pair of bonding electrons., , , C2HO, 5, , H, , H, , H– C— C– H, H, , s+, s–, C2H5O — H, , H, , H — CH, , CH2, –, Brs, , Br, , C2H5OH + H — C, , , , C — H + Br, , H, , E 1 CB React ion : Mechanism does operate under rather special circumstances 1, 1, 1 Trifluoro–2, 2, dichloroethane for instance, undergoes base-catalysed exchange of -hydrogen atom with the solvent deuterium, ethoxide faster than dehydrofluorination., , CF3—CHCl2, , C2H5OD, , , , CF3—C, solw, , Cl, Cl, , Fast, , CF3—CDCl2, , CF2=CCl2, E 1 Reacti on : The Uni molecular Mechani sm :, The main feature of this mechanism is that under the influence of solvation forces, the electron attracting, group ("leaving group") breaks away along with the bonding electrons. The resultant carbonium ion subsequently loses a proton to the solvent or to some other proton acceptor., H, H, , slow, –C—C–, –C—C–, +X, , X, , H, –C—C–, , , fast, , –C, , C– + H, , H, –C C–, X, The reaction thus has two stages of which the first is the rate-determining step and as a result the reaction, rate depends only on the concentration of the first reactant., Rate = k, , Or ientat i on i n elimi nat i on react ions :, The elimination reactions of unsymmetrical substrates usually yields mixtures of all possible products. There, are two empirical rules governing the orientation in these reactions., (a), , The saytzeff Rule : It states that neutral substrates (alkyl halides or sulphonates) capable of forming, a double bond in either direction of the chain preferably yield that alkene in which there is greater, number of alkyl groups attached to the double bond. This rule applies to E1 reaction and to most, of E2 reactions. The following examples are typical.
Page 49 :
SOLVED EXA MPLES, Ex.1, , If CH3, , CH, , CHO gives up proton the most-stable carbanion would be, , CH3, , (A), , CH3, , CH2, CH, , CHO, , (B), , CH3, , Sol., , CHO (C), , CH2, , C, , CHO, , CH3, , (D) All the above, , This carbanion shows resonance :, H3C, , H3C, C, , CH, , O, , Ans.( C ), C, , CH, , O, , H3C, , H3C, , Ex.2, , CH3, CH, , The most stable intermediate is :, , , •, , (A) BrCH 2CH 2 C H 2, , (B) CH3– CH– C H 2, , , , (C) CH 3– CH –CH 2Br, , (D) All are equally stable, , Br, , Ans. (C), Ex.3, , Which of the following is a true statement, (A) RCOOH is more stable than RCOO–, (B) RCOO– is more stable than RCOOH, (C) RCOOH and RCOO– do not show resonating structures, (D) All of the above, , Sol., , RCOO– is more stable than RCOOH due to resonance., , Ex.4, , The electron attracting species in the following is –, (A) –CN, , Sol., , (B) –NH 2, , Ans.( B ), , (C) –NHR, , (D) –NHCOR, , The groups having double or triple bonds are electron attracting. Hence–CN is electron attracting and in, rest of the species N–atom has lone pair of electrons., , Ex.5, , Which carbanion is less stable than the other three (A) CH2 NO2, , Sol., , (B) CH2 CHO, , Compound :, O, , (D) CH3, Ans.( C ), , O, , on removal of proton gives a carbanion. The most stable carbanion should be, O, , (A), Sol., , (C) CH2 CH3, , Due to + I effect of CH3 group CH2 CH3 is less stable., O, , Ex.6, , Ans.(A ), , O, , (B), , O, , O, , (C), , O, , (D) All the above, , In this case negatively charged carbon is present between two electron attracting groups. As such it is a, stable carbanion., , Ans.( C )
Page 50 :
Ex.7, , +, , , , CH2 O H CH2= O –H. The stability order of above structure is I, , II, , (A) I > II, nd, , (B) II > I, , (C) I = II, , Sol., , In II, , Ex.8, , Bond fission (I) CH4 C H 3 + H and (II) C H 3, , (D) None of these, , structure carbon completes its octet. Hence it is more stable, , , , , , , , , (A) C H 3 and :CH2 both are free radicals, , Ans. (B), , , :CH2 + H about (I) and (II) the false statement is, (B) Bond dissociation energy II > I, , , , (C) C H 3 and :CH2 have similar geometry, , (D) Both bond fission are homolytic, , Ans. (C), , Sol., , The geometry of CH3 is trigonal planar while the geometry of :CH2 is linear and trigonal plannar.., , Ex.9, , Which of the following alkene is minimum stable., (A) R 2 C, , CR 2, , (B) R—CH, , Sol., , (D) due to less substituted alkene., , Ex.10, , Which is maximum acidic :, , OH, , (A), , (C) R—CH, , (C), , Cl, , CH—R, , (D) R—CH, , OH, , OH, (B), , NO2, , CR 2, , OH, , (D), , CH3, , CH 2, , Ans. (A), , OCH3