Page 1 :
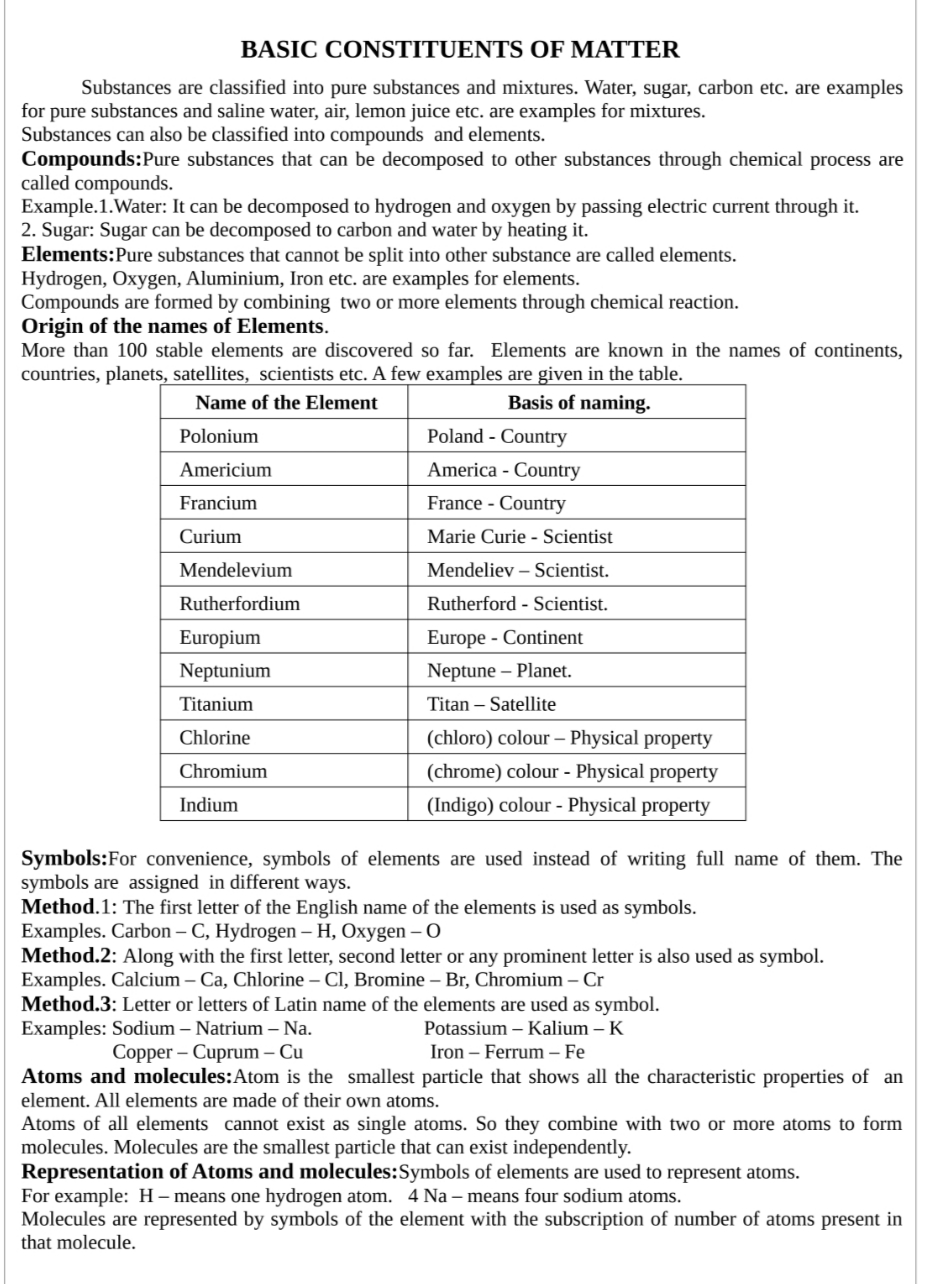
BASIC CONSTITUENTS OF MATTER, , Substances are classified into pure substances and mixtures. Water, sugar, carbon etc. are examples, for pure substances and saline water, air, lemon juice etc. are examples for mixtures., Substances can also be classified into compounds and elements., Compounds:Pure substances that can be decomposed to other substances through chemical process are, called compounds., Example.1.Water: It can be decomposed to hydrogen and oxygen by passing electric current through it., 2. Sugar: Sugar can be decomposed to carbon and water by heating it., Elements: Pure substances that cannot be split into other substance are called elements., Hydrogen, Oxygen, Aluminium, Iron etc. are examples for elements., Compounds are formed by combining two or more elements through chemical reaction., Origin of the names of Elements., More than 100 stable elements are discovered so far. Elements are known in the names of continents,, countries, planets, satellites, scientists etc. A few examples are given in the table., , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , Name of the Element Basis of naming., Polonium Poland - Country, Americium America - Country, Francium France - Country, Curium Marie Curie - Scientist, Mendelevium Mendeliev — Scientist., Rutherfordium Rutherford - Scientist., Europium Europe - Continent, Neptunium Neptune — Planet., Titanium Titan — Satellite, Chlorine (chloro) colour — Physical property, Chromium (chrome) colour - Physical property, Indium (Indigo) colour - Physical property, , , , , , Symbols:For convenience, symbols of elements are used instead of writing full name of them. The, symbols are assigned in different ways., Method.1: The first letter of the English name of the elements is used as symbols., Examples. Carbon — C, Hydrogen — H, Oxygen -O, Method.2: Along with the first letter, second letter or any prominent letter is also used as symbol., Examples. Calcium — Ca, Chlorine — Cl, Bromine — Br, Chromium — Cr, Method.3: Letter or letters of Latin name of the elements are used as symbol., Examples: Sodium — Natrium — Na. Potassium — Kalium — K, , Copper — Cuprum — Cu Iron — Ferrum — Fe, Atoms and molecules:Atom is the smallest particle that shows all the characteristic properties of an, element. All elements are made of their own atoms., Atoms of all elements cannot exist as single atoms. So they combine with two or more atoms to form, molecules. Molecules are the smallest particle that can exist independently., Representation of Atoms and molecules:Symbols of elements are used to represent atoms., For example: H — means one hydrogen atom. 4 Na-— means four sodium atoms., Molecules are represented by symbols of the element with the subscription of number of atoms present in, that molecule.