Page 1 :
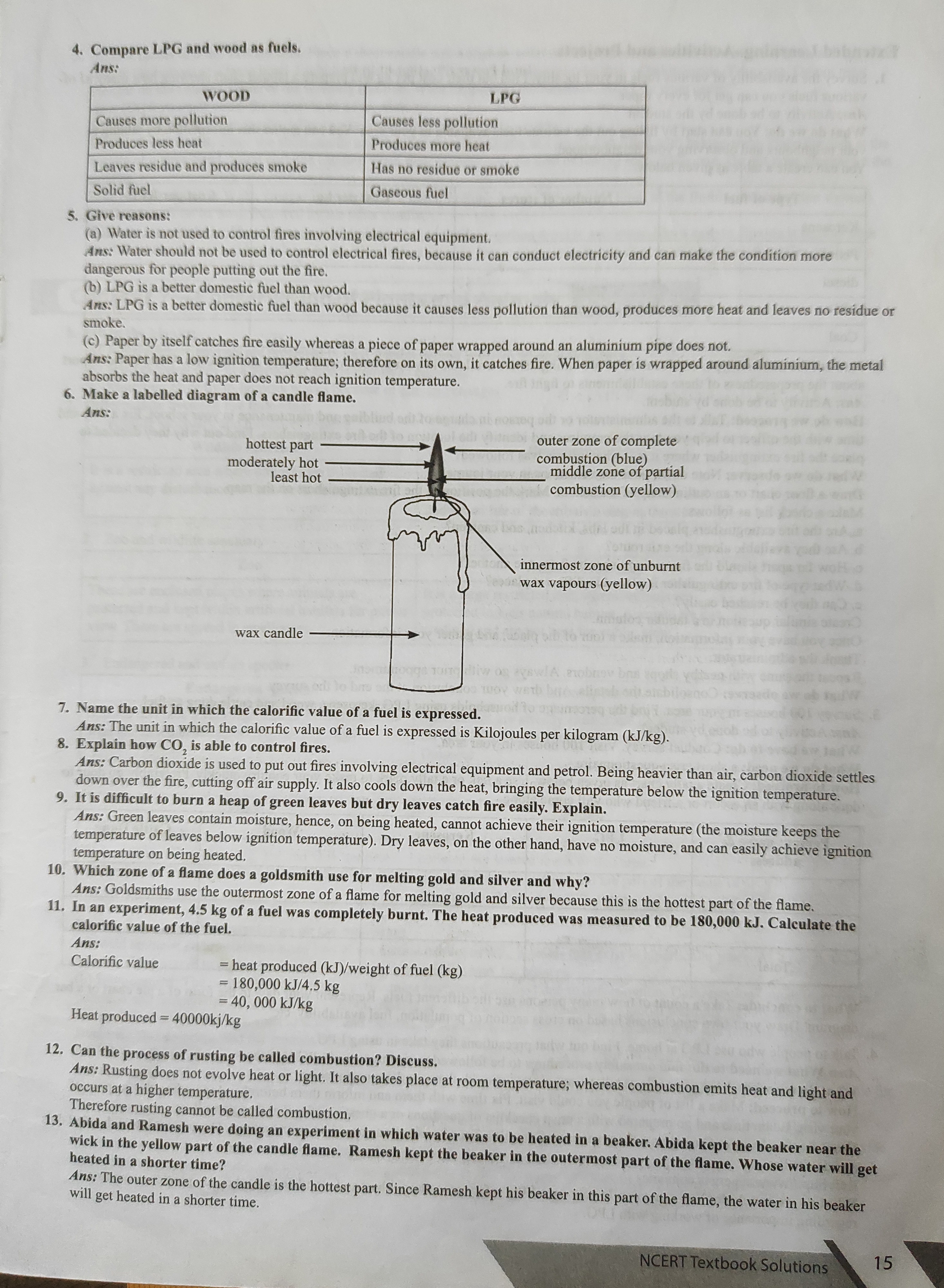
4, Compare LPG and wood as fuels., Ans:, , WOOD _ — — LPG, , Causes more pollution Causes less pollution, , , , , , , , , , , , Produces less heat ., Leaves residue and produces smoke, Solid fuel, , Produces more heat, , , , Has no residue or smoke, , , , Gaseous fuel, , , , §,. Give reasons:, , (a) Water is not used to control fires involving electrical equipment. = oat, , Ans: Water should not be used to control electrical fires, because it can conduct electricity and can make the condition more, dangerous for people putting out the fire,, , (b) LPG is a better domestic fuel than wood. ;, , Ans: LPG is a better domestic fuel than wood because it causes less pollution than wood, produces more heat and leaves no residue or, smoke. ae, , (c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not. a, Ans: Paper has a low ignition temperature; therefore on its own, it catches fire. When paper is wrapped around aluminium, the meta, absorbs the heat and paper does not reach ignition temperature., , . Make a labelled diagram of a candle flame., , Ans:, , outer zone of complete, , ombustion (blue), Serdar ene en, , - combustion (yellow), , hottest part ————————_», moderately hot, least hot, , , , , , innermost zone of unburnt, ~ wax vapours (yellow), , wax candle, , - Name the unit in which the calorific value of a fuel is expressed., , Ans: The unit in which the calorific value of a fuel is expressed is Kilojoules per kilogram (kJ/kg)., , - Explain how CO, is able to control fires., , Ans: Carbon dioxide is used to put out fires involving electrical equipment and petrol. Being heavier than air, carbon dioxide settles, , down over the fire, cutting off air supply. It also cools down the heat, bringing the temperature below the ignition temperature., , - It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain. _, , 10., , EE, , 12,, , 13, , Ans: Green leayes contain moisture, hence, on being heated, cannot achieve their ignition temperature (the moisture keeps the, temperature of leaves below ignition temperature). Dry leaves, on the other hand, have no moisture, and can easily achieve ignition, , temperature on being heated,, Which zone of a flame does a goldsmith use for melting gold and silver and why?, Ans: Goldsmiths use the outermost zone of a flame for melting gold and silver because this is the hottest part of the flame,, , In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the, calorific value of the fuel. ;, , , , , , , , Ans:, , Calorific value = heat produced (kJ)/weight of fuel (kg), = 180,000 kJ/4.5 kg, = 40, 000 kJ/kg, , Heat produced = 40000kj/kg, , Can the process of rusting be called combustion? Discuss., , Ans: Rusting does not evolve heat or light. It also takes place at room temperature; whereas combustion emits heat and, occurs at a higher temperature., , Therefore rusting cannot be called combustion,, , aoe ore nen? were doing an experiment in which water was to be heated in a beaker. Abida kept the beake:, , ic © yellow part of the candle flame. Ramesh kept the beaker in the outermost pa ], heated in a shorter time? i ea i, Ans: The outer zone of the candle is the hottest, , part. Since Ramesh kept his beaker in this the flame, the water i, will get heated in a shorter time. P part of the flame, the water in