Page 1 :
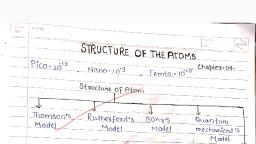
Activity-1 , To understand the structure of an atom, scientists developed different atomic models., Thomson's Model of the Atom , This atomic model was proposed by J.J. Thomson in 1898. This model was commonly called the plum pudding model, referring to the way the fruit pieces are distributed throughout a plum pudding. , According to this model:, An atom is considered to be a sphere of uniform positive charge and electrons are embedded in it., The total mass of the atom is considered to be uniformly distributed through out the atom., The negative and the positive charges are supposed to balance out and the atom as a whole is electrically neutral.
Page 2 :
A more familiar example that represents Thomson's atomic model is watermelon. The positive charge is spread throughout the atom like the red part of watermelon. The black seeds distributed through out the red part represents electrons. , Thomson's model was modified by one of his students. , What is the reason for its modification? , The reason was that some of the experiments carried out by Rutherford, gave results, which were not in favour of Thomson's model., What are the limitations of J.J. Thomson’s model of the atom?
Page 3 :
Rutherford's alpha particles scattering experiment, Ernest Rutherford was a scientist born in New Zealand. In 1909, he did some experiments using gold foil and alpha particles. , Alpha particles consist of two protons and two neutrons bound together. Since they do not have any electrons, they are positively charged with two units of charge. , There is a source of the fast moving alpha particles which have a considerable amount of energy., The stream of alpha particles is directed towards a very thin gold foil., The gold foil was placed inside a detector in such a way that the detector would show a flash of light when an alpha particle struck it. The entire arrangement was kept in a vacuum chamber.
Page 4 :
When the alpha particle hit the foil, Rutherford expected that they all would be deflected by the positive charge spread evenly throughout the gold atoms., But, it was found that most of the alpha particles passed straight through the atoms without any deflection, some particles deflected in small angles. , Only very few particles were deflected through large angles and a very small number of particles were reflected right back. , This led Rutherford to think about new atomic model.
Page 5 :
Rutherford concluded from the alpha particles scattering experiment that : , (i) Most of the space inside the atom is empty because most of the alpha particles passed through the gold foil. , (ii) A very small fraction of alpha particles that were deflected right back indicated that they had met a very large positive charge and mass which repelled the charge on the alpha particle. So, all the positive charge must be concentrated in a very small space within the atom., What were the three major observations Rutherford made in the gold foil experiment?
Page 6 :
On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features: , i) All the positively charged particles in an atom formed a small dense centre, called the nucleus of the atom. The electrons were not a part of nucleus., ii) He also proposed that the negatively charged electrons revolve around the nucleus in well-defined orbits. Rutherford's model is sometimes referred to as the planetary model because the motion of the electrons around the nucleus resembles the motion of the planets around the Sun. , iii) The size of the nucleus is very small as compared to the size of the atom., Nucleus, Orbit
Page 7 :
Limitations of Rutherford's atomic model , The electron is attracted by the proton in the nucleus. Even in circular motion around the nucleus, the electron should has an acceleration., An accelerated charged particle moving in a circular path would always radiate energy continuously. , Thus, the revolving electron would lose energy continuously and get directed towards the positively charged nucleus and eventually crash into the nucleus., If this is true, the atoms would become highly unstable and the matter would not exist in the form that we see it now. But we know that atoms are stable., Sketch Rutherford’s atomic model. Why Rutherford’s model of the atom is called the planetary model?