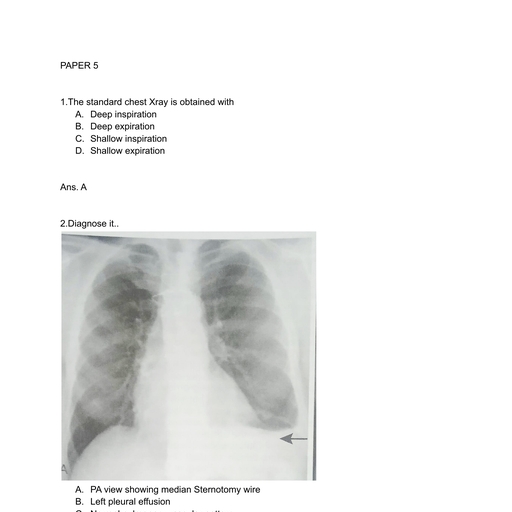
Page 1 : CHAPTER, , 37, , The Fetal Heart, Elizabeth R. Stamm and Julia A. Drose, , SUMMARY OF KEY POINTS, • Moderate to severe congenital heart disease (CHD) occurs, in approximately 6 per 1000 live births., • The incidence of CHD is much higher in the fetal, population, estimated to be at least 15 per 1000., • The incidence of CHD is higher with positive family, history, monochorionic twins, and in vitro, fertilization., • Most fetuses with CHD have no known risk factors., , • Fetal echocardiography is a safe and effective method of, detecting CHD., • Early detection of CHD may have a substantial impact on, care and prognosis, allowing intrauterine therapy, carefully, planned delivery, and parental counseling., • Advancements in surgical techniques and perioperative, management have significantly improved prognosis in, many cases., , CHAPTER OUTLINE, NORMAL FETAL CARDIAC, ANATOMY AND SCANNING, TECHNIQUES, STRUCTURAL ANOMALIES, Atrial Septal Defect, Ventricular Septal Defect, Atrioventricular Septal Defect, Ebstein Anomaly, Hypoplastic Right Ventricle, Hypoplastic Left Heart Syndrome, , S, , Univentricular Heart, Tetralogy of Fallot, Truncus Arteriosus, Double-Outlet Right Ventricle, Transposition of Great Arteries, Anomalous Pulmonary Venous Return, Coarctation of Aorta, Aortic Stenosis, Pulmonic Stenosis, Cardiosplenic Syndromes, , onographic evaluation of the fetal heart can identify cardiac, abnormalities that affect obstetric care in a variety of ways,, including mode of delivery, location of delivery, intrauterine, therapy, parental reassurance, and opportunity for termination., Congenital heart disease (CHD) is a significant problem, with, an incidence of moderate and severe CHD of approximately 6, per 1000 live births. If trivial lesions such as tiny muscular, ventricular septal defects (VSDs) are included, the incidence, is as high as 75 per 1000 live births.1 It is more difficult to, determine the exact incidence of CHD in the fetal population., One large study suggested that CHD occurs in at least 15 per, 1000 fetuses.2 More than 20% of perinatal deaths caused by, congenital malformations are the result of a congenital heart, defect.3 In 85% of CHD cases, both environmental and genetic, factors are involved (Table 37.1).4-7 The remaining 15% of cardiac, anomalies are associated with a single gene or chromosomal, abnormality.5 The risk of CHD increases to 2% to 3% with an, affected sibling and to approximately 10% with two affected, siblings or an affected mother—although the incidence is variable, depending on the type of CHD in the affected relative.5,8 The, , 1270, , Cardiac Tumors, Cardiomyopathy, Ectopia Cordis, ARRHYTHMIAS, Premature Atrial and Ventricular, Contractions, Tachycardia, Bradycardia, Congenital Heart Block, , risk to offspring of affected mothers is substantially higher than, for those with affected fathers, suggesting that cytoplasmic, inheritance may play a role in the genetics of CHD (Tables 37.2, and 37.3). Only 50% of recurrent heart lesions are of the same, type as the previously diagnosed defect.9, Extracardiac malformations occur in 25%,10 and chromosomal anomalies occur in 13% of live-born neonates with, CHD.11-13 About 50% of fetuses with nonimmune hydrops and, cardiac anomalies have a chromosomal anomaly, and 10% will, have extracardiac anomalies.14 Hydrops in the setting of CHD, is predictive of a very poor prognosis., Although the most common indications for formal fetal, echocardiography are family history of CHD and fetal arrhythmia, the majority of these fetuses will have normal hearts. The, highest incidence of CHD occurs in patients referred because, of an abnormal four-chamber view, fetal hydrops, or significant, polyhydramnios on a routine obstetric ultrasound.15,16 A routine, ultrasound examination with a suspected fetal cardiac anomaly, is associated with a 50% to 69% risk of CHD in live-born, infants.17,18 Monochorionic twins are at a higher risk for CHD,, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 4 : CHAPTER 37, , SUGGESTED RISK, (%), , Fibroelastosis, Ventricular septal defect, Patent ductus arteriosus, Atrioventricular septal defect, Atrial septal defect, Tetralogy of Fallot, Pulmonary stenosis, Coarctation of aorta, Aortic stenosis, Hypoplastic left heart, Transposition, Tricuspid atresia, Ebstein anomaly, Truncus, Pulmonary atresia, , If One, Sibling, , If Two, Siblings, , 4, 3, 3, 3, 2.5, 2.5, 2, 2, 2, 2, 1.5, 1, 1, 1, 1, , 12, 10, 10, 10, 8, 8, 6, 6, 6, 6, 5, 3, 3, 3, 3, , a, , Combined data published during two decades from European and, North American populations., With permission from Nora J.252, , TABLE 37.3 Suggested Offspring, Recurrence Risk (%) for Congenital Heart, Defects Given One Affected Parent, AFFECTED PARENT, Defect, Aortic stenosis, Atrial septal defect, Atrioventricular septal defect, Coarctation of aorta, Pulmonary stenosis, Tetralogy of Fallot, Ventricular septal defect, , 1273, , affords parents whenever normal cardiac anatomy and function, are documented in a fetus at risk., , TABLE 37.2 Recurrence Risks, in Siblings for Any Congenital, Heart Defecta, , Defect, , The Fetal Heart, , Father, , Mother, , 3, 1.5, 1, 2, 2, 1.5, 2, , 13-18, 4-4.5, 14, 4, 4-6.5, 2.5, 6-10, , With permission from Nora J.252, , with literature suggesting a ninefold increase in the incidence, of CHD in monochorionic-diamniotic twin gestations.19 Lastly,, there appears to be a slightly increased risk of CHD with in vitro, fertilization (IVF), although at least some of this increased risk, appears to be related to the increased incidence of twin pregnancies associated with IVF.20 Most fetuses with CHD have no known, risk factors, which underscores the importance of a meticulous, evaluation of the four-chamber heart views and outflow tracts, on all routine obstetric ultrasound examinations. When severe, structural cardiac anomalies are identified before viability, termination may be offered. Certainly, one of the most important, aspects of fetal echocardiography is the psychological relief it, , NORMAL FETAL CARDIAC ANATOMY, AND SCANNING TECHNIQUES, The fetal heart is similar to that of the adult, with several anatomic, and physiologic differences. The long axis of the fetal heart is, perpendicular to the long axis of the body, such that a transverse, section through the fetal thorax demonstrates the four cardiac, chambers in a single view. The adult heart, in contrast, is obliquely, oriented with its long axis along a line between the left hip and, the right shoulder. The four-chamber view is important because, 10% to 96% of structural anomalies are detectable on this, view.21-26, , Common Indications for Fetal, Echocardiography, Abnormal heart on routine ultrasound, Hydrops, Polyhydramnios, Fetal arrhythmia, Fetal chromosomal anomalies, Fetal extracardiac anomalies, Family history (congenital heart disease [CHD], syndromes, associated with CHD), Maternal disease (diabetes, collagen vascular,, phenylketonuria), Maternal infection (rubella), Teratogen exposure, Increased nuchal translucency on first-trimester screening, In vitro fertilization, Monochorionic twins, Monitoring response to intrauterine therapy, Monitoring fetus at risk for decompensation (persistent, tachyarrhythmia, hydrops), , Cardiac axis and position are normally such that the apex of, the heart points to the left and the bulk of the heart is in the left, side of the chest (Fig. 37.1A). This is levocardia. In mesocardia, the heart is central with the apex pointing anteriorly. In dextrocardia the apex is directed rightward, and the heart is primarily, in the right side of the chest. This abnormality must be distinguished from dextroposition (Fig. 37.1B), in which the heart, maintains a normal axis but is displaced to the right by an external, process, such as a left chest mass or pleural effusion. Abnormal, cardiac axis is associated with a 50% mortality and abnormal, cardiac position with an 81% mortality.27, The fetal cardiovascular system contains several unique shunts:, the ductus venosus, foramen ovale, and ductus arteriosus (Fig., 37.2). Antenatally, the placenta rather than the lungs is the fetus’s, sole source of oxygen. Oxygenated blood leaves the placenta, through the umbilical vein and travels through the hepatic, vasculature and ductus venosus to the inferior vena cava (IVC), and then into the fetal right atrium. As a result of increased, velocity, blood entering the IVC via the ductus venosus is shunted, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 5 : 1274, , PART IV, , Obstetric and Fetal Sonography, , A, , B, FIG. 37.1 Heart Position and Axis. (A) Normal position and axis of the heart. The heart is predominantly in the left side of the chest, with, the apex of the heart pointing leftward. Dual-screen image shows the stomach also on the left side. (B) Dextroposition of fetal heart caused by, a large, congenital pulmonary airway malformation. Transverse image through the fetal chest shows the heart displaced to the right, but the, apex (arrow) remaining leftward. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; S, stomach. Dual-screen image shows the, stomach on the correct left side., , across the foramen ovale to the left atrium and then into the left, ventricle, the aorta, and the fetal brain. Poorly oxygenated blood, from the superior vena cava (SVC) also enters the right atrium,, and mixes with the blood entering from the IVC, but continues, to the right ventricle and pulmonary artery. Most of this blood, is directed through the ductus arteriosus into the descending, aorta. Thus these shunts function so that the majority of output, from both ventricles enters the systemic circulation, rather than, a substantial portion entering the pulmonary circulation, as in, the adult. Blood that enters the pulmonary vasculature via the, pulmonary artery is returned to the left atrium by the four, pulmonary veins. From the left atrium it enters the left ventricle, and ultimately the descending aorta, returning to the placenta, via the iliac and umbilical arteries. Normal values for measurements of the fetal heart and great vessels are shown in Figs. 37.3, and 37.4., Fetal echocardiography is best accomplished at 18 to 22, weeks of gestation.28 Before 18 weeks, resolution is frequently, , limited by the small size of the fetal heart. After 22 weeks the, examination may be compromised by progressive ossification, of the fetal skull, spine, and long bones; the relatively smaller, amniotic fluid volume; and unaccommodating fetal position., Importantly, some congenital cardiac abnormalities progress in, utero and may be subtle or unrecognizable at or before 22 weeks, but more obvious closer to term.29,30 Tachyarrhythmias may not, become apparent until the third trimester.31 In some cases, firsttrimester evaluation of the fetal heart may be accomplished with, transvaginal ultrasound as early as 11 to 14 weeks.32-34 More, recently, diagnostic results have been possible with a transabdominal approach at 11 to 13 weeks.34 However, first-trimester, fetal echocardiography is limited and should be considered an, adjunct to second-trimester evaluation, not a replacement., Scanning the fetal heart requires a systematic approach,, beginning with determination of the position of the fetus within, the uterus and the heart within the fetal chest. A transverse view, through the fetal thorax above the level of the diaphragm, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 6 : CHAPTER 37, , Ductus, arteriosus, , Foramen, ovale, , Ductus, venosus, , Umbilical, vein, , Umbilical, arteries, , Placenta, FIG. 37.2 Diagram of Fetal Shunts. Blood from the umbilical vein, is shunted through the ductus venosus to the right atrium and then, across the foramen ovale to the left atrium. Fetal cardiac output from, the right side of the heart is shunted to the descending aorta through, the ductus arteriosus., , demonstrates four cardiac chambers. Four-chamber views, can be obtained with the angle of insonation parallel to the, interventricular septum (apical four-chamber view; Fig. 37.5A,, Video 37.1) or perpendicular to the septum (subcostal fourchamber view; Fig. 37.5B, Video 37.2). In a four-chamber view, the echogenic foraminal flap of the foramen ovale can be observed, moving into the left atrium. The two superior pulmonary veins, may be seen entering the spherical left atrium. The atrioventricular valves are visible in the four-chamber view. The septal, leaflet of the tricuspid valve inserts slightly more apically on the, interventricular septum than the anterior leaflet of the mitral, valve. The left ventricle has a relatively smooth inner wall, and, a more elongated shape than the right ventricle. In the normal, heart, the left ventricle is the apex forming ventricle. The internal, surface of the right ventricle is coarse, particularly near the apex,, where the moderator band of the trabecula septomarginalis, is frequently recognized as a small, brightly echogenic focus., This helps identify the morphologic right ventricle., From the subcostal four-chamber view, angling the transducer, toward the fetus’s right shoulder permits evaluation of the continuity of the left ventricle with the ascending aorta (Fig. 37.6). Further, angulation in the same direction shows the right ventricle in, continuity with the pulmonary artery (Fig. 37.7, Videos 37.3, and 37.4). The diameter of the pulmonary artery is approximately, 9% larger than that of the aorta between 14 and 42 weeks. The, measured differences in these vessels and with M-mode versus, , The Fetal Heart, , 1275, , two-dimensional (2-D) imaging are negligible (2%-5%) for both, the pulmonary artery and the aorta.35 Further rightward rotation, produces a sagittal view of the fetal thorax and a short-axis view, of the ventricles (Fig. 37.8, Video 37.5). Angulation toward the, left fetal shoulder from this view shows the aorta as a central, circle, with the pulmonary artery draping anteriorly and to the, left (Fig. 37.9)., The apical four-chamber view may also be used as a starting, point when evaluating normal cardiac anatomy. Yagel and colleagues36 described a series of planes arising from the apical, four-chamber view, all accomplished by moving the transducer, in a cephalad direction. A slight cephalad advancement will show, an apical five-chamber view, which is useful in assessing continuity of the ascending aorta with the left ventricle (Fig. 37.10)., Continued cephalad movement should result in visualization of, the bifurcating pulmonary artery and its relationship to the right, ventricle. A three-vessel and trachea view should be visualized, next (Fig. 37.11, Video 37.6). This view allows evaluation of, the main pulmonary artery–ductus arteriosus confluence, the, transverse aortic arch, and the SVC. Comparison of vessel size,, confirmation of vessel presence, and determination of blood, flow direction with color Doppler can all be accomplished at, this level. In addition, appropriate location of both great vessels, to the left of the trachea can be confirmed.36 Returning to a, sagittal plane of the fetus, directing the transducer from the fetal, left shoulder to the right hemithorax demonstrates the distinctive, candy-cane shape of the aortic arch (Fig. 37.12, Videos 37.7 and, 37.8). The three major vessels to the head and neck and the, ductus arteriosus may be seen. The aortic arch should not be, confused with the ductal arch (Fig. 37.13), which is formed by, the right ventricular outflow tract, pulmonary artery, and ductus, arteriosus. The ductal arch is broader and flatter than the aortic, arch. Lastly, sliding the transducer to the right while maintaining, a sagittal plane on the fetus should allow visualization of the, IVC and SVC entering the right atrium., M-mode echocardiography provides a 2-D image of motion, over time. It is useful in evaluating heart rate, chamber size, wall, thickness, and wall motion (Fig. 37.14). Simultaneous M-mode, imaging through an atrium and ventricle is helpful in analyzing, arrhythmias (Fig. 37.15). Chamber size and function should be, evaluated at the level of the atrioventricular (A-V) valves.37, Spectral Doppler ultrasound evaluation of the fetal heart, can be used to determine the velocity of flow through the vessels, or valves (Fig. 37.16) and to evaluate regurgitant flow through, the valves of the heart (Fig. 37.17). Variation in flow velocity, may reflect structural or functional cardiac abnormalities. For, example, a stenotic A-V valve will be associated with an abnormal, flow pattern through the affected valve. Spectral Doppler ultrasound is useful in assessing the functional significance of structural, abnormalities and arrhythmias., Color Doppler ultrasound permits a rapid interrogation of, flow patterns within the heart and great vessels (Fig. 37.18),, allowing functional and structural abnormalities to be more, rapidly characterized. For example, valvular stenosis is clearly, demonstrated with color Doppler ultrasound, as is reversed flow, through insufficient valves or in the great vessels. Color Doppler, ultrasound often reduces the amount of time required for spectral, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 7 : 1276, , A, , B, , PART IV, , Obstetric and Fetal Sonography, , C, , D, , E, FIG. 37.3 Cardiac Dimensions. (A) Left ventricular internal dimension versus gestational age. y = 0.049x − 0.262. (B) Right ventricular internal, dimension versus gestational age. y = 0.045x − 0.228. (C) Posterior left ventricular wall thickness versus gestational age. y = 0.012x − 0.063., (D) Septal thickness versus gestational age. y = 0.012x − 0.088. (E) Left atrial internal dimension versus gestational age. y = 0.040x − 0.214. In, each graph, the 95% confidence limits represent twice the standard error of the mean. (With permission from Allan LD, Joseph MC, Boyd EG,, Campbell S, Tynan M. M-mode echocardiography in the developing human fetus. Br Heart J. 1982;47[6]:573-583.250), , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 8 : CHAPTER 37, , The Fetal Heart, , 1277, , Doppler ultrasound evaluation of the heart, particularly in the, setting of complex cardiac anomalies.38-41 Subtle lesions such as, small VSDs may be more reliably and easily identified with the, use of color flow Doppler ultrasound., The utility of several advanced technologies has been reported, in the evaluation of the fetal heart, including three-dimensional, (3-D) and four-dimensional (4-D) ultrasound, tissue Doppler, imaging, strain and strain rate imaging, as well as fetal magnetocardiography and cardiovascular magnetic resonance, imaging (MRI).42-44 However, many of these require specialized, transducers or other equipment, sophisticated algorithms and, specialized technical expertise. Additionally, limited resolution, and cardiac motion are still considered major disadvantages in, some settings.45, , A, , STRUCTURAL ANOMALIES, Atrial Septal Defect, B, FIG. 37.4 Diameter of Aortic Root and Pulmonary Artery., (A) Diameter of aortic root versus gestational age. (B) Diameter of, pulmonary artery (PA) versus gestational age. Norms and confidence, limits for echocardiographic measurements. (With permission from Cartier, MS, Davidoff A, Warneke LA, et al. The normal diameter of the fetal, aorta and pulmonary artery: echocardiographic evaluation in utero. Am, J Roentgenol. 1987;149[5]:1003-1007.35), , A, , An atrial septal defect (ASD) results from an error in the amount, of tissue resorbed or deposited in the interatrial septum. It is, the fifth most common form of CHD and is the most common, form in adult patients.46,47 ASDs occur in 1 per 1500 live births48,49, and comprise 6.7% of CHD in live-born infants.46 ASDs occur, twice as often in females as males.50,51 ASDs are associated with, a variety of cardiac, extracardiac, and chromosomal abnormalities., ASDs can be classified by embryogenesis, size, or relationship, to the fossa ovalis., , B, , FIG. 37.5 Four-Chamber View of Heart. (A) Apical four-chamber view shows the interatrial and interventricular septa parallel to the angle of, insonation. See also Video 37.1. (B) Subcostal four-chamber view shows the interatrial and interventricular septa perpendicular to the angle of, insonation. See also Video 37.2. LA, Left ventricle; LV, left ventricle; RA, right atrium; RV, right ventricle., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 9 : 1278, , PART IV, , Obstetric and Fetal Sonography, , FIG. 37.6 Continuity of aorta (AO) with left ventricle (LV). RV, right, ventricle. See also Videos 37.3 and 37.4., , FIG. 37.8 Short-Axis View of Ventricles. Anterior right ventricle, (RV) is normally slightly larger than the left ventricle (LV). See also Video, 37.5., , PV, PA, RVOT, AO, RA, , LA, , FF, , FIG. 37.7 Continuity of pulmonary artery (PA) with right ventricle, (RV). See also Video 37.3 and Video 37.4., , SP, , FIG. 37.9 Short-Axis View of Great Vessels. Aorta (AO) in center, with pulmonary artery (PA) draping anteriorly. FF, Foraminal flap; LA,, left atrium; PV, pulmonic valve; RA, right atrium; RVOT, right ventricular, outflow tract; SP, spine., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 10 : CHAPTER 37, , LV, , RV, , AO, , The Fetal Heart, , 1279, , LS, LC, , LA, , A, , I, RA, , SP, , FIG. 37.10 Apical five-chamber view shows continuity of the aorta, (A) with the left ventricle (LV). LA, Left atrium; RA, right atrium; RV,, right ventricle; SP, spine., , FIG. 37.12 Normal Aortic Arch. Sagittal view shows the rounded,, “candy-cane” appearance of aortic arch and the head and neck vessels, arising from it. See also Video 37.7 and Video 37.8. AO, Descending, aorta; I, innominate artery; LC, left carotid artery; LS, left subclavian, artery., , PA, A, , D, , LA, AO, , FIG. 37.13 Normal Ductal Arch. Sagittal view shows pulmonary, artery (PA) draping over the aorta (A) and joining the ductus arteriosus, (D), which then joins the descending aorta (AO). LA, Left atrium., , FIG. 37.11 Three-vessel and trachea view shows the correct orientation of the main pulmonary artery–ductus arteriosus confluence (P), the, transverse aortic arch (A), and the superior vena cava (S). This view also, shows the two great vessels correctly positioned on the left side of the, trachea (T). See also Video 37.6. SP, Spine., , Embryologically, between the fourth and sixth weeks of, gestation the primitive atrium is divided into right and left halves., The septum primum, a crescent-shaped membrane, develops, along the cephalad portion of the atrium and grows caudally, toward the endocardial cushions. The space between these two, structures, termed the ostium primum, disappears when the, septum primum fuses with the endocardial cushion. Before, complete fusion, however, multiple small fenestrations develop, in the septum primum, coalescing to form the ostium, secundum., , A second crescent-shaped membrane subsequently develops, just to the right of the septum primum. As this membrane grows, toward the endocardial cushion, it partially covers the ostium, secundum. Its crescent-shaped lower border never entirely fuses, with the endocardial cushion, leaving an opening, the foramen, ovale (Fig. 37.19)., Ostium secundum ASDs make up more than 80% of all, ASDs and generally occur in isolation. This ASD is caused by, excessive resorption of the septum primum (foraminal flap) or, by inadequate growth of the septum secundum (Fig. 37.20A)., The ostium primum ASD is the second most common type and, is located low in the atrial septum, near the atrioventricular, (A-V) valves. Although the ostium primum ASD may occur, alone, it is more frequently associated with a more complex, congenital cardiac anomaly, the atrioventricular septal defect, (AVSD) (Fig. 37.20B)., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 11 : 1280, , PART IV, , Obstetric and Fetal Sonography, , A, , 1 cm, , 1.0 sec, , AL, , RV, Open, , Closed, , AO, F F, LA, , PL, , B, , TV, IVS, MV, , C, , FIG. 37.14 M-Mode Echocardiography. (A) M-mode tracing through the right atrium (RA) and the left ventricle (LV) showing normal atrial, contractions followed by normal ventricular contractions. LA, Left atrium; RV, right ventricle. (B) M-mode tracing through the aortic root shows the, aortic valve opening and closing. The foraminal flap (F) can be seen opening into the left atrium (LA). AL, Anterior leaflet of aortic valve; AO, aorta;, PL, posterior leaflet of aortic valve; RV, right ventricle. (C) M-mode tracing shows opening and closing of the mitral valve (MV) and tricuspid valve, (TV). IVS, Interventricular septum., , The sinus venosus ASD is a rare defect that can be divided, into two types: (1) sinus venosus ASD of the SVC, with the, defect adjacent to the SVC, and (2) sinus venosus ASD of the, IVC, with the defect adjacent to the IVC. The first type is often, associated with anomalous pulmonary venous return (APVR), (Fig. 37.20C). Coronary sinus ASDs, located at the ostium of, the coronary sinus in the right atrium, may also occur but are, exceedingly rare., The prenatal sonographic diagnosis of ASD is difficult because, the normal patent foramen ovale, which allows blood to flow, from the right to the left atrium in utero, itself represents an, ASD. It can be difficult to distinguish a small, pathologic ASD, from the normal patent foramen ovale. The foraminal flap, or, septum primum, is clearly visualized on the four-chamber view., It has a “loose pocket” configuration, appearing either circular, or linear in shape as it opens into the left atrium52,53 (Fig. 37.21)., The septum secundum, which is thick and relatively stationary,, makes up the majority of the atrial septum. The foramen ovale, is an opening in the septum secundum. The septum secundum, , and foramen ovale are well visualized in the four-chamber, views. The maximal size of the normal foramen ovale differs, by 1 mm or less from the aortic root diameter at all gestational, ages.54 An ostium secundum ASD appears as a larger than, expected defect in the central portion of the atrial septum near, the foramen ovale. Alternatively, it can appear as a deficient, foraminal flap., If the lowest portion of the atrial septum (just adjacent to the, A-V valves) is deficient, an ostium primum defect should be, suspected (Fig. 37.22). Color Doppler ultrasound may be helpful, in the diagnosis of larger ASDs. However, small ASDs are commonly obscured by the normal flow through the patent foramen, ovale.55,56, A large right-to-left shunt is physiologic in utero, and thus, an ASD usually does not compromise the fetus hemodynamically., After birth, the shunt may cause right ventricular overload and, pulmonary hypertension. Spontaneous closure of an ASD will, occur in approximately two-thirds of patients.57 Patients with, small ASDs may remain asymptomatic into their 50s.58, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 12 : The Fetal Heart, , CHAPTER 37, , 1281, , RA, RV, LV, RV, , LA, , Vel 519 cm/s, PG 108 mmHg, , RA, , R, , V, , V, , A, , V, , PV, , V, , A, , V, , PA–, , A, , V, , A, , V, , A, , A, , T, , A, , FIG. 37.15 Using M-Mode Echocardiography to Analyze an, Arrhythmia: Conducted Premature Atrial Contractions. The cursor, is placed simultaneously through the left ventricle (LV) and right atrium, (RA). The M-mode tracing shows normal atrial beats (A) followed by a, premature atrial contraction (PA). The ventricles show normal ventricular, contraction (V) following each atrial beat and a premature beat (PV), following the premature atrial contraction. LA, Left atrium; RV, right, ventricle., , RV, , LV, , RA, , LA, SP, , A, E, , A, E, , A, E, , A, E, , A, E, , A, E, , R, , A, E, , FIG. 37.16 Spectral Doppler Ultrasound Used to Interrogate a, Normal Mitral Valve. Spectral Doppler sample volume is placed distal, to the mitral valve in the left ventricle (LV). A normal mitral valve waveform, is appreciated above the baseline, showing the normal early diastolic, (E) and atrial contraction (A) wave points. LA, Left atrium; RA, right, atrium; RV, right ventricle; SP, spine., , R, , T, , R, , T, , R, , T, , FIG. 37.17 Tricuspid Insufficiency. Spectral Doppler sample volume, is placed proximal to the tricuspid valve in the right atrium (RA). The, regurgitant flow (R) can be seen above the baseline. This implies that, the valve has not closed completely during systole, and therefore blood, flow is retrograde into the right atrium. RV, Right ventricle; T, tricuspid, valve., , Ventricular Septal Defect, Isolated VSD is the most common cardiac anomaly, accounting, for 30% of heart defects diagnosed in live-born infants and 9.7%, diagnosed in utero.46,47,59 VSDs are associated with other cardiac, anomalies in 50% of cases.60 Of the structural cardiac defects,, VSDs have the highest recurrence rate and the highest association, with teratogen exposure. They are classified according to their, position in the interventricular septum (Fig. 37.23) as membranous or muscular VSD (inlet, trabecular, outlet).60, About 80% of VSDs occur in the membranous portion of the, septum.61 However, because most membranous defects also involve, a portion of the muscular septum, they are usually referred to, as perimembranous defects. The subcostal four-chamber view, provides optimal evaluation of the interventricular septum. At, sonography, a VSD appears as an area of discontinuity in the, interventricular septum. When the defect is small, this diagnosis, is problematic, and at least one-third of VSDs are missed on the, four-chamber view.21,55,62-66 Color Doppler imaging may improve, the diagnostic accuracy for VSD. However, most are missed on, fetal echocardiography.55,66,67 Small VSDs not detectable on grayscale echocardiography may be documented with color Doppler, ultrasound in some cases39 (Fig. 37.24). In the setting of an isolated, VSD, color Doppler ultrasound imaging typically shows bidirectional interventricular shunting, with a systolic right-to-left, shunt and a late diastolic left-to-right shunt., The prognosis for an infant with an isolated VSD is excellent,, and many such defects go undetected. The rate of spontaneous, closure of isolated muscular VSDs by 5 years of life is much, higher (65%) than that for isolated perimembranous VSDs (20%).68, Overall about 40% of VSDs spontaneously close in the first year, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 14 : CHAPTER 37, , RA, , The Fetal Heart, , 1283, , RV, , LA, , LV, , A, , B, , RV, , RA, , LV, , LA, , C, , FIG. 37.21 Foraminal Flap and Foramen Ovale. (A) Linear, appearance of the foraminal flap (arrow) as it enters the left atrium, (LA). (B) Circular appearance of the foraminal flap (arrow) entering, the LA. (C) Color Doppler ultrasound in a subcostal four-chamber view, shows normal flow through the foramen ovale from the LA to the, right atrium (RA). LV, Left ventricle; RV, right ventricle., , Pulmonary valve, , Outlet, Tricuspid, valve, RA, Membranous, RV, , LA, , Inlet, , Trabecular, , LV, FIG. 37.23 Interventricular Septum Viewed From Right Ventricle. The membranous septum and the three portions of the muscular, septum (inlet, outlet, and trabecular) are demonstrated. Ventricular septal, defects may occur in any of these locations., , FIG. 37.22 Four-chamber view shows an ostium primum atrial, septal defect (arrow) in a fetus with an atrioventricular septal defect., LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle., Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 15 : 1284, , Obstetric and Fetal Sonography, , PART IV, , of life, and 60% resolve by 5 years of age.69-71 However, large, defects detected in the fetus are associated with an 84% mortality.72, Concurrent cardiac, extracardiac, and chromosomal anomalies, (trisomy 13, 18, 21, and 22) are associated with a worse, prognosis., VSDs may be extremely difficult to diagnose in utero, particularly when small in size. In addition, many small VSDs, will close in utero or shortly after birth. A “pseudo” VSD in, the membranous portion of the septum may be appreciated, when evaluating the interventricular septum from an apical, , LA, RA, , four-chamber view. This occurs when the angle of insonation is, parallel to the septum, causing an artifactual dropout of the thin,, membranous septum., , Atrioventricular Septal Defect, AVSD refers to a spectrum of cardiac abnormalities involving, various degrees of deficiency of the interatrial and interventricular, septa and of the mitral and tricuspid valves. These defects arise, when the endocardial cushions fail to fuse properly and were, previously called endocardial cushion defects or A-V canal, defects. Almost two-thirds of fetuses with AVSD have additional, cardiac anomalies.73-75 About one-third are associated with left, atrial isomerism (both atria anatomically resemble the left),, and of these the majority of affected fetuses have complete heart, block.72,73 Chromosomal (especially trisomy 21) or extracardiac, anomalies are associated in 78% of AVSDs.72, Embryologically, in the primitive heart, the common atrium, and ventricle communicate through the A-V canal. Development, of the endocardial cushion results in division of the single, large, A-V canal into two separate orifices, separating the atria from, the ventricles (Fig. 37.25). The interatrial and interventricular, septa develop concurrently, eventually dividing the single atrium, and ventricle into right and left portions. When the endocardial, cushions fail to fuse properly, normal development of the mitral, and tricuspid valves cannot occur, and an AVSD results, (Fig. 37.26)., AVSDs are divided into complete and partial or incomplete, forms.76 In both, the A-V valves are abnormal. In complete AVSD, a single, multileaflet valve is present, whereas in incomplete, AVSD two of the leaflets (bridging leaflets) are connected by a, narrow strip of tissue, resulting in the appearance of two valve, , LV, S, RV, , FIG. 37.24 Muscular Ventricular Septal Defect (VSD). Subcostal, four-chamber view using color Doppler ultrasound shows a muscular, VSD (arrow) across the interventricular septum (S). LA, Left atrium; LV,, left ventricle; RA, right atrium; RV, right ventricle., , 4 wk, , 5 wk, , Septum primum, , Septum secundum, , Atrioventricular, canal, , Ostium secundum, Septum primum, fused to, endocardial cushions, , Endocardial, cushion, Common ventricle, , A, , Interventricular, septum, , Fused endocardial, cushions, , B, , Interventricular, septum, , 8 wk, Septum secundum, Ostium secundum, Septum primum, Mitral valve, , C, , Tricuspid valve, , FIG. 37.25 Normal Development of Endocardial Cushions. (A) In the fourth week the endocardial cushions divide the atrioventricular canal, into two orifices. (B) By the fifth week the communication between the atria, the ostium secundum, is smaller. The ventricular septum has grown,, almost obliterating the communication between the ventricles. (C) At 8 weeks, complete development of the endocardial cushions and atrioventricular, valves results in four distinct cardiac chambers., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 16 : CHAPTER 37, Normal tricuspid and mitral valves, Aortic leaflet, , Inferior leaflet, , Mural leaflet, , A, , 1285, , Partial atrioventricular septal defect, , Anterosuperior, leaflet, Septal leaflet, , The Fetal Heart, , RT, , LT, , “Cleft”, , Tricuspid Mitral, valve, valve, , B, , Complete atrioventricular septal defect, Anterosuperior, leaflet, Right mural, leaflet, , C, , Anterior, bridging leaflet, RT, , LT, , Left mural, leaflet, Posterior, bridging leaflet, , FIG. 37.26 Valve Leaflet Morphology. (A) Normal heart. (B) Partial atrioventricular septal defect (AVSD). (C) Complete AVSD. LT, Left abnormal, valve; RT, right abnormal valve., , RA, RA, , RV, , LA, , LA, RV, , LV, , A, , LV, , B, , FIG. 37.27 Atrioventricular Septal Defect (AVSD). (A) Apical four-chamber view shows absent atrial septum, resulting in a single large atrium, (RA-LA). A ventricular septal defect is visible between the left ventricle (LV) and right ventricle (RV). A single, multileaflet atrioventricular valve is, also appreciated. (B) Apical four-chamber view shows color Doppler ultrasound filling the atrioventricular septal defect. See also Video 37.9. LA,, Left atrium; RA, right atrium., , orifices. Complete AVSD has variable amounts of deficient tissue, in the atrial and ventricular septa. The incomplete form is associated with an ostium primum ASD. At fetal echocardiography,, 97% of AVSDs are complete, although after birth only 69% are, complete.67,72 The fetal incidence of AVSD is four times greater, than that in the live-born population, indicating a high incidence, of in utero demise.46,47,77, AVSDs are considered balanced when the A-V junction is, connected to both the right and the left ventricle, such that blood, flow is relatively evenly distributed. If this connection exists with, primarily one ventricle, such as in the setting of a hypoplastic, left ventricle, it is termed an unbalanced AVSD., , Sonographically, a defect in the atrial or ventricular septum, with an associated single abnormal A-V valve is visible in a, four-chamber view (Fig. 37.27, Video 37.9). The abnormal valve, should be suspected when the normal offset of the A-V valves, is not visualized or when only a single A-V valve is appreciated, in a short-axis view. Demonstration of two A-V valve orifices, allows for differentiation between complete and incomplete forms, of AVSD.62, Color Doppler ultrasound demonstrates an open area of flow, across the AVSD and the abnormal A-V valve. Color Doppler, ultrasound imaging is particularly useful in the detection of, valvular insufficiency.78 Holosystolic valvular insufficiency is, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 17 : 1286, , PART IV, , Obstetric and Fetal Sonography, , Ebstein anomaly, , RA, , RV, , LA, LV, , RA, , Mitral, valve, LA, , Tricuspid, valve, , Anterior, , A, , B, , FIG. 37.28 Ebstein Anomaly. (A) Diagram showing the tricuspid valve apically displaced, resulting in an enlarged right atrium (RA) and a small,, functional right ventricle (RV). (B) Gray-scale image shows tricuspid valve (arrow) displaced inferiorly, resulting in an “atrialized” RV and enlarged, RA. See also Video 37.12. LA, Left atrium; LV, left ventricle., , closely associated with fetal hydrops and a worsening prognosis.79, Frequently, a left ventricular–to–right atrial jet can be identified, across the ostium primum defect before the onset of holosystolic, valvular insufficiency.38 Cardiac malformations associated with, AVSD include septum secundum ASD, hypoplastic left heart, syndrome (HLHS), valvular pulmonary stenosis, coarctation of, the aorta, and tetralogy of Fallot (TOF). A meta-analysis of, published cases of AVSD diagnosed prenatally confirmed that, chromosomal anomalies are common, occurring in 25% to 58%, of affected fetuses.80 Therefore karyotyping is indicated. Associated, extracardiac anomalies are common, including omphalocele,, duodenal atresia, tracheoesophageal atresia, facial clefts, cystic, hygroma, neural tube defects, and multicystic kidneys.80, The fetus with an AVSD and associated defects has a poor, prognosis. When hydrops is present, few survive the neonatal, period.81 Despite advances in pediatric cardiothoracic surgery,, the overall outcome for antenatally diagnosed AVSD remains, variable, with many studies reporting 5-year to 15-year survival, rates below 50%.80,82 Others have reported excellent long-term, results with newer surgical techniques such as the two-patch, technique with early complete cleft closure for complete AVSD, repair.83, , Ebstein Anomaly, Ebstein anomaly is characterized by inferior displacement of the, tricuspid valve, frequently with tethered attachments of the, leaflets, tricuspid dysplasia, and right ventricular dysplasia84-88, (Fig. 37.28, Video 37.12). Ebstein anomaly makes up approximately 7% of cardiac anomalies in the fetal population and has, an incidence of 0.5% to 1% in high-risk populations.64,89,90 It, occurs in approximately 1 per 20,000 live births.91, Early data from biased retrospective studies suggested that, lithium use during pregnancy was associated with an estimated, , 500-fold increase in the incidence of Ebstein anomaly in, exposed fetuses.91-96 It is now clear that the increased risk is, less than 2%.45,97 Ebstein anomaly may be associated with a, variety of structural cardiovascular defects, particularly pulmonary atresia or stenosis,98 arrhythmias, and chromosomal, anomalies.86,99-102, Ebstein anomaly is readily detected in utero.99,103 The sonographic diagnosis rests on recognition of apical displacement of the, tricuspid valve into the right ventricle, an enlarged right atrium, containing a portion of the “atrialized” right ventricle, and a, reduction in size of the functional right ventricle. Differential, diagnosis includes tricuspid valvular dysplasia, Uhl anomaly,, and idiopathic right atrial enlargement, but none of these has, an inferiorly displaced tricuspid valve, the most reliable sign of, Ebstein anomaly. Ebstein anomaly is one of the few structural, defects that may cause substantial cardiac dysfunction in utero,, frequently with cardiomegaly, hydrops, and tachyarrhythmias.86, Examination with spectral and color Doppler ultrasound is, helpful in demonstrating tricuspid valve regurgitation, which, causes further enlargement of the right atrium and ventricle.104, Tethered distal attachments of the tricuspid valve, marked right, atrial enlargement, and left ventricular compression with narrowing of the pulmonary outflow tract are all associated with a, poor prognosis.86 Fetuses diagnosed with Ebstein anomaly and, tricuspid dysplasia have a poor prognosis, with an overall perinatal, mortality (fetal demise or death before neonatal discharge), of 45%.105, Arrhythmias, particularly supraventricular tachycardias, (SVTs), are common with Ebstein anomaly and can further, compromise the fetus. Overall, the 3-month mortality rate for, patients diagnosed in utero is 80%.86,103 Surgical correction of, Ebstein anomaly in young children is associated with a low, mortality rate and an excellent quality of life.106-108 Because clinical, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 18 : The Fetal Heart, , CHAPTER 37, , RV, , LV, , RV, , RA, , LV, RA, , 1287, , LA, , LA, , SP, SP, , FIG. 37.29 Hypoplastic Right Ventricle. Apical four-chamber view, shows small, right ventricular chamber (RV). See also Video 37.11. LA,, Left atrium; LV, left ventricle; RA, right atrium; SP, spine., , presentation, treatment options, and prognosis are inconsistent,, case-by-case management is variable.109, , Hypoplastic Right Ventricle, In general, hypoplastic right ventricle occurs secondary to, pulmonary atresia with intact interventricular septum. It has, an incidence of 1.1% among stillbirths.46 Tricuspid atresia may, be associated with a hypoplastic right ventricle, but this is not, as common.110 Pathophysiologically, hypoplasia of the right, ventricle develops because of a reduction in blood flow secondary, to inflow impedance from tricuspid atresia or outflow impedance, from pulmonary arterial atresia. Typical sonographic findings, include a small, hypertrophic right ventricle and a small or absent, pulmonary artery110 (Fig. 37.29, Video 37.11). Spectral Doppler, ultrasound may be helpful in demonstrating decreased flow, through the tricuspid valve or pulmonary artery. Congestive, heart failure and hydrops may develop from tricuspid regurgitation. After birth, closure of the ductus arteriosus frequently results, in neonatal death. Prognosis improves with preoperative prostaglandin infusion to maintain the patency of the ductus.111, , Hypoplastic Left Heart Syndrome, In hypoplastic left heart syndrome, the left ventricular cavity, is pathologically reduced in size. HLHS constitutes approximately, 7% to 9% of all congenital cardiac lesions.112 It has a 2 : 1 male, predominance and a recurrence risk of 0.5%.112,113 The small left, ventricle results from decreased blood flow into or out of the, left ventricle. The primary abnormalities include aortic atresia,, aortic stenosis, and mitral valve atresia. It is associated with, coarctation of the aorta in 80% of cases.114 The primary sonographic feature of HLHS is a small left ventricle (Fig. 37.30,, Video 37.10). The mitral valve is typically hypoplastic or atretic,, , FIG. 37.30 Hypoplastic Left Heart Syndrome. The left atrium (LA), and left ventricle (LV) are small. See also Video 37.10. RA, Right atrium;, RV, right ventricle; SP, spine., , as is the aorta.115 Color Doppler ultrasound is extremely helpful, in the setting of HLHS, usually demonstrating the absence of, flow through the mitral and aortic valves.39, Three decades ago this syndrome had an extremely poor, prognosis, with 25% mortality in the first week of life and most, untreated infants dying within 6 weeks.116 Comfort care was, provided, but little could be done to prolong survival. Currently, it is expected that up to 70% of newborns with HLHS may reach, adulthood owing to advancements in surgical techniques,, perioperative management, and postoperative care.117 Prenatal, diagnosis of HLHS is beneficial for preventing ductal shock and, keeping affected infants stable in the preoperative stage.118-120, Monophasic blood flow across the mitral valve, restricted or, absent flow through the foramen ovale, and retrograde flow, through the aorta are all considered poor prognostic signs in, utero. Despite significant advancements in medical and surgical, management over the past 30 years, follow-up studies indicate, that children with HLHS often experience major developmental, delays121 and decreased exercise performance, even after heart, transplantation.122 A recent meta-analysis found that although, deficits remain, substantial improvement in neurodevelopment, has occurred over the past 20 years in patients with surgically, corrected HLHS.123, , Univentricular Heart, In univentricular heart, two atria empty into a single ventricle,, via two A-V valves or a common A-V valve. Univentricular heart, is rare, accounting for approximately 2% of CHD.47 It results, from a failure of the interventricular septum to develop. The, single chamber has a left ventricular morphology in 85% of, cases.124 Associated cardiac anomalies are common,125 with, asplenia or polysplenia occurring in 13%.126 Sonographically,, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 19 : 1288, , PART IV, , Obstetric and Fetal Sonography, , RV, AO, IVS, LV, , FIG. 37.31 Tetralogy of Fallot. The aorta (AO) overrides both the, right ventricle (RV) and the left ventricle (LV). A ventricular septal defect, (arrows) is also appreciated. See also Video 37.13. IVS, Interventricular, septum., , a single ventricle with absence of the interventricular septum is, seen. Doppler ultrasound examination is helpful in determining, if a normal outflow tract is present. A nonfunctioning, rudimentary accessory ventricle may be present in some cases., Differential diagnosis includes a large VSD and hypoplastic right, or left ventricle., Patients with outflow tract stenosis have a poor prognosis.127, Death is typically caused by congestive heart failure or arrhythmia.77 Pulmonary artery banding and shunts yield a 70% 5-year, survival rate. Ventricular septation has a postoperative survival, rate of approximately 56%.125,128 More recent literature indicates, that there continues to be a high mortality rate for univentricular, heart after surgical correction, with an 8-year survival after, modified Blalock-Taussig shunt of only 68%.129 After a Glenn, bidirectional cavopulmonary connection, 8-year survival is, slightly higher at 74%.130, , Tetralogy of Fallot, Tetralogy of Fallot consists of (1) VSD, (2) overriding aorta,, (3) hypertrophy of the right ventricle, and (4) stenosis of the, right ventricular outflow tract (Fig. 37.31, Video 37.13). It accounts, for 5% to 10% of CHD in live births46 and is associated with a, variety of cardiac, extracardiac, and chromosomal anomalies.72, A study of 129 fetuses diagnosed in utero with TOF reported, additional cardiac anomalies in 57%, extracardiac anomalies in, 50%, and chromosomal anomalies in 49%. The first trimester, nuchal translucency was above the 95th percentile in 47% of, fetuses.131, TOF occurs when the conus septum is located too far, anteriorly, thus dividing the conus into a smaller, anterior right, ventricular portion and a larger posterior part. Closure of the, interventricular septum is incomplete, causing the aorta to, override both ventricles.132 The VSD typically occurs in the, perimembranous portion of the septum. Right ventricular, hypertrophy rarely occurs in utero, but the overriding aorta is, reliably seen.133,134 Pulmonary atresia or stenosis, or a dilated, pulmonary artery secondary to absence of the valve, may be, appreciated. The diagnosis of TOF has been made before 15, , weeks’ gestation using transvaginal ultrasound.33 Color Doppler, imaging is helpful in making the diagnosis of TOF.135, The newborn with the classic form of TOF who has pulmonary, stenosis rather than pulmonary atresia is typically asymptomatic, at birth but develops cyanosis and a murmur in the first weeks, of life. Early primary repair of TOF is routinely performed with, a low surgical mortality and postoperative morbidity.136 Typical, cases of TOF are repaired at 4 to 6 months of age, with close to, 90% survival at 1 year.137 Patients surviving early surgery (before, 5 years old) have a 32-year survival of 90%.138 The presence of, congestive heart failure in the fetus or newborn with TOF is, associated with 17% to 41% mortality.139,140, , Truncus Arteriosus, Truncus arteriosus accounts for 1.3% of fetal cardiac anomalies, and is characterized by a single large vessel arising from the base, of the heart. This vessel supplies the coronary arteries and the, pulmonary and systemic circulations. Aortic anomalies occur, in 20% and noncardiac anomalies in 48% of patients with truncus, arteriosus.141 In almost all patients a VSD is present. The truncal, valve may have two to six cusps and in general overrides the, ventricular septum. Four types of truncus arteriosus have been, identified by Collett and Edwards,142 as follows:, • Type I has a pulmonary artery that bifurcates into right and, left branches after it arises from the ascending portion of the, truncal vessel., • Type II has right and left pulmonary arteries arising separately, from the posterior truncus., • Type III has pulmonary arteries that arise from the sides of, the proximal truncus., • Type IV has systemic collateral vessels from the descending, aorta as the source of flow., The single, large truncal artery with overriding ventricular, septum and an associated VSD is identified on four-chamber and, outflow tract views (Fig. 37.32). This anomaly has been diagnosed, as early as 13 weeks.34 Color Doppler imaging is particularly, helpful in the setting of truncus arteriosus because it facilitates, accurate localization of the pulmonary arteries and rapidly detects, truncal valvular insufficiency. In the 1980s, prognosis was poor,, with an overall mortality of 70%.141 More recent studies have, indicated that 10-year to 20-year survival is excellent for infants, undergoing complete repair of truncus arteriosus.143 However,, these patients continue to experience significant comorbidities, throughout childhood, with significant deficits in exercise tolerance and overall functional status.144, , Double-Outlet Right Ventricle, Double-outlet right ventricle (DORV) represents less than 1%, of all CHD and occurs when more than 50% of both the aorta, and the pulmonary artery arise from the right ventricle.145,146, DORV is classified into the following three types:, • Aorta posterior and to the right of the pulmonary artery, • Aorta and pulmonary artery parallel, with the aorta to the, right (Taussig-Bing type), • Aorta and pulmonary artery parallel, with the aorta anterior, and to the left, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 20 : CHAPTER 37, , The Fetal Heart, , 1289, , RV, PA, RV, , AO, TA, , LV, , SP, FIG. 37.33 Double-Outlet Right Ventricle. The aorta (AO) and, pulmonary artery (PA) both arise from the right ventricle (RV) in a parallel, fashion., FIG. 37.32 Truncus Arteriosus. The single truncal artery (TA) overrides both the right ventricle (RV) and left ventricle (LV). A ventricular, septal defect (arrow) is present. No pulmonary artery was seen, helping, to differentiate from tetralogy of Fallot. SP, Spine., , DORV is associated with other cardiac defects (particularly, VSD), various extracardiac defects, fetal chromosomal anomalies,, maternal diabetes, and maternal alcohol consumption.5,146,147 With, surgical intervention, 10-year survival as high as 97% has been, reported.145 A more recent study reported near 94% overall 5-year, survival after surgical correction.148 When extracardiac or, chromosomal anomalies are present, prognosis is poor, with, 69% mortality when the diagnosis of DORV is made in utero.147, Sonographically, the aorta and pulmonary artery arise predominantly from the right ventricle (Fig. 37.33). Differential diagnosis, includes transposition of the great vessels and TOF., , A, P, , Transposition of Great Arteries, Transposition of the great arteries (TGA) is subdivided into two, types: (1) complete or dextrotransposition (D-TGA) in 80%, and (2) congenitally corrected or levotransposition (L-TGA), in 20% of fetuses with transposition. In both types, ventriculoarterial discordance is present. (The aorta arises from the right, ventricle, and the pulmonary artery arises from the left ventricle.), Complete transposition (D-TGA) is defined as atrioventricular, concordance (atria and ventricles are correctly paired) with, ventriculoarterial (V-A) discordance (Fig. 37.34). It comprises, 5.5% of heart disease in the fetal population.47 D-TGA is also, classified into two types, depending on the absence (70%) or, presence of a VSD. A variety of cardiac anomalies are associated, with D-TGA, including pulmonic stenosis, which rarely occurs, in the absence of a VSD. In 8% of cases, other organ systems, are involved. Chromosomal anomalies are not commonly associated with TGA., , FIG. 37.34 Complete Transposition of Great Arteries. The aorta, (A) is anterior to the pulmonary artery (P). This abnormal arrangement, results in both vessels running parallel to each other in this short-axis, view., , In D-TGA, the aorta arises from the right ventricle, receives, systemic blood, and returns it to the systemic circulation. The, pulmonary artery arises from the left ventricle, receives pulmonary, venous blood, and returns it to the lungs. In general, the aortic, root lies anterior and slightly to the right of the pulmonary outflow, tract. With closure of the ductus arteriosus and foramen ovale, after birth, this condition is incompatible with life unless an, associated shunt allows mixing of the separate right and left, circulations., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 21 : 1290, , Obstetric and Fetal Sonography, , PART IV, , RV, , LA, , LV, RA, , FIG. 37.35 Congenitally Corrected Transposition of Great Arteries. An apical four-chamber view shows the morphologic right ventricle, (RV) and morphologic left ventricle (LV) located on the incorrect sides, of the heart. This is evidenced by identifying the atrioventricular valve, leaflets inserting (arrow) on the left side of the heart in a more apical, location than the right-sided atrioventricular valve leaflet insertion., LA, Left atrium; RA, right atrium., , Sonographic diagnosis depends on demonstrating that the, great vessels exit the heart in parallel, rather than crossing in, the normal fashion. This is optimally seen in a long-axis or, short-axis view of the great vessels. A three-vessel view is also, useful because only one great vessel (aorta) is usually visualized, at this level, in this setting., Most neonates with D-TGA require immediate treatment., Initially, a temporizing shunt may be created before definitive, treatment, frequently with the arterial switch procedure. With, surgical intervention, 12-month survival can be expected in, 80%.149 Early intervention (within 3 days of life) when using the, arterial switch operation is optimal and is associated with an, operative mortality below 2%. Major morbidity and cost increase, daily thereafter.150, Corrected transposition (L-TGA) is characterized by A-V, discordance with V-A discordance (Fig. 37.35). It comprises 1%, of CHD and 20% of cases of fetal TGA.47 The aorta, which arises, from the left-sided, morphologic right ventricle, is anterior and, to the left of the pulmonary artery. The pulmonary artery arises, from the right-sided, morphologic left ventricle. VSD and, pulmonic stenosis occur in approximately half the cases.124,151, Malformation and inferior displacement of the morphologic, tricuspid valve may be present. Pathophysiologically, the flow, of blood through the heart to the pulmonic and systemic circulations is normal, even though the morphologic right ventricle is, on the left and the morphologic left ventricle is on the right., The antenatal sonographic diagnosis rests on demonstrating, a parallel arrangement to the great vessels, similar to D-TGA., Differentiating D-TGA from L-TGA entails identification of the, morphologic right and left ventricles. The moderator band will, be seen on the anatomic left side. In addition, the tricuspid valve, will be situated on the anatomic left side, so its more apical septal, , leaflet should be identified. Associated cardiac defects are common, and diverse, including VSD, pulmonary stenosis or atresia, ASD,, DORV, tricuspid valve anomalies, dextrocardia, mesocardia, and, situs inversus. Fetal A-V block is common with TGA.152, In the absence of associated cardiac anomalies, patients, with corrected TGA may remain asymptomatic throughout their, lives., , Anomalous Pulmonary Venous Return, APVR can be divided into two subgroups: total anomalous, pulmonary venous return (TAPVR), in which none of the, pulmonary veins drains into the left atrium, and partial anomalous pulmonary venous return (PAPVR), in which at least one, of the pulmonary veins has an anomalous connection. TAPVR, constitutes 2.3% of cases of CHD.153,154 The four types of anomalous, pathways are as follows:, 1. The pulmonary veins drain into a vertical vein that empties, into the innominate vein and then into the SVC., 2. The pulmonary veins drain into the coronary sinus and then, into the right atrium., 3. The pulmonary veins drain directly into the right atrium., 4. The pulmonary vein drains into the portal vein and into the, IVC via the ductus venosus., Embryologically, TAPVR is thought to result from failure of, obliteration of the normal connections between the primitive, pulmonary vein and the splanchnic, umbilical, vitelline, and, cardinal veins. TAPVR is associated with AVSDs and polysplenia, and asplenia syndromes., The antenatal sonographic diagnosis of TAPVR is difficult, because the anomalous veins are generally extremely small and, variable in their course. Often the first sign of APVR is mild, right ventricular and pulmonary artery prominence, in which, case a careful search for the four normal pulmonary veins should, be undertaken.155 This can be difficult because the two inferior, pulmonary veins are usually more difficult to visualize than the, two superior veins, even in a normal fetal heart. Color and spectral, Doppler ultrasound are helpful in documenting the normal, pulmonary venous anatomy and in detecting and following the, anomalous connections (Fig. 37.36)., The diagnosis of TAPVR is suspected when no pulmonary, veins are seen entering the left atrium (Fig. 37.37). A small left, atrium resulting from decreased blood return and lack of normal, incorporation of the common pulmonary vein into the left atrium, is also suggestive of TAPVR. Approximately one-third of patients, with TAPVR have associated cardiac anomalies.156 Right atrial, isomerism is common. Associated extracardiac anomalies include, gut malrotation and midline liver and stomach.157,158 TAPVR, causes minimal hemodynamic disturbance in utero, although, hydrops occasionally results. Left untreated, the majority of infants, die before 1 year of age.133 Although PAPVR has also been, diagnosed in utero, the diagnosis is more difficult and can be, made only when pulmonary veins are seen entering the left atrium, as well as the right atrium or an accessory pathway to the right, atrium.159 APVR is associated with high morbidity and mortality,, largely because of the high incidence of additional cardiac, anomalies.157,159 Surgical correction of TAPVR is associated with, an operative mortality of nearly 20%.160, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 22 : CHAPTER 37, , The Fetal Heart, , RA, , 1291, , RV, , LA, LV, LV, , LA, , RV, , P, , RA, P, D, , A, , S, , D, , S, , D, , S, , D, , S, , B, , FIG. 37.36 Normal Pulmonary Venous Anatomy. (A) Four-chamber view using color Doppler ultrasound shows two superior pulmonary, veins (P) entering the left atrium (LA). (B) Subcostal four-chamber view using pulsed Doppler ultrasound shows normal waveform and direction of, pulmonary venous flow into the LA. D, Diastolic peak; LV, left ventricle; RA, right ventricle; RV, right ventricle; S, systolic peak., , LV, , RV, P, , LA, , RA, , P, P, P, , FIG. 37.37 Total Anomalous Pulmonary Venous Return. Apical, four-chamber view shows anomalous insertion of all four pulmonary, veins (P) into the right atrium (RA). LA, Left atrium; LV, left ventricle;, RV, right ventricle., , Coarctation of Aorta, Aortic coarctation is a narrowing of the aortic lumen, usually, occurring between the insertion of the ductus arteriosus and, the left subclavian artery. Its severity ranges from a slight narrowing at the distal end of the arch to severe hypoplasia of the, entire arch. Of fetuses with CHD, coarctation has an incidence, of 6.8%.47 Almost 80% of the cases are associated with other, , cardiac anomalies, including abnormal aortic valve (bicuspid or, stenotic), VSD, DORV, and AVSD. Chromosomal abnormalities, occur in 5%, and almost 5% of coarctations are associated with, maternal diabetes.59,161 Coarctations are present in approximately, 20% of individuals with Turner syndrome (45X).5, Three embryologic theories have been proposed to explain, the origin of coarctation of the aorta: (1) a primary developmental, defect with failure of connection of the fourth and sixth aortic, arches with the descending aorta162; (2) aberrant ductal tissue, at the level of the aortic arch163,164; and (3) decreased blood flow, through the aortic isthmus.165, Sonographic detection of coarctation is difficult.25 Ventricular, size discrepancy with a prominent right ventricle and relatively, small left ventricle,55,165 with a right-to-left ventricle diameter, ratio greater than 2 standard deviations (SDs) above the norm,166,167, suggests coarctation of the aorta. Likewise, a discrepancy in, pulmonary artery–to–ascending aorta diameter that falls greater, than 2 SDs above the normal ratio of 1.18 to 0.06167 is suggestive, of coarctation.166 Color Doppler ultrasound is useful in identifying, the area of narrowing. Spectral Doppler ultrasound may detect, increased velocity distal to the narrowed segment (Fig. 37.38)., Many coarctations do not become evident until closure of the, ductus arteriosus at birth. In addition, infants with coarctation, of the aorta may not develop clinical or echocardiographic signs, of coarctation until 6 to 12 weeks after closure of the ductus, arteriosus. If coarctation of the aorta is suspected on fetal, echocardiogram, the infant should be followed to at least 1 year, of age.168 Although isolated coarctation has a good prognosis,, 39% mortality is reported when associated anomalies are, present.169 Treatment is usually accomplished with angioplasty, or stenting with good results.170, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 23 : 1292, , PART IV, , Obstetric and Fetal Sonography, , A, , B, , FIG. 37.38 Coarctation of Aorta. (A) Gray-scale image showing narrowing of the aortic arch (arrow) at the level of the isthmus. (B) Spectral, Doppler tracing shows increased velocity and bidirectional blood flow through the aortic arch., , Aortic Stenosis, Aortic stenosis is a stricture or obstruction of the ventricular, outflow tract occurring in 5.2% of newborns.46 Aortic stenosis, is divided into supravalvular, valvular, and subvalvular types., The supravalvular type of aortic stenosis has not been reported, in utero. Valvular aortic stenosis is more frequent in males and, is associated with a bicuspid aortic valve and chromosomal, abnormalities.171 Subvalvular aortic stenosis is associated with, inherited disorders, asymmetrical septal hypertrophy (ASH),, and hypertrophic obstructive cardiomyopathy. Infants of diabetic, mothers may have a transient form of left ventricular outflow, tract obstruction secondary to ASH., Thickening of the aortic valve, poststenotic dilation of the, aorta, and ventricular enlargement are clues to valvular aortic, stenosis. In addition, real-time evaluation of the aortic valve, may show it persisting, as opposed to moving normally in and, out of the field of view. Thickening of the interventricular septum, may be seen in subvalvular aortic stenosis. In all cases, increased, velocity through the aortic valve will be identified on pulsed, Doppler ultrasound. Early-onset aortic stenosis results in endocardial fibroelastosis (EF) and hypoplastic left ventricle.172, Aortic stenosis progresses in utero and may not be apparent, on early (<16 weeks) fetal echocardiograms. In some cases these, defects may not be apparent until after birth.29 A mortality rate, of 23% in the first year of life is reported with aortic stenosis.173, Prognosis has improved with appropriate surgery, with mortality, of 1.9% to 9%.174,175 In some cases, severe aortic stenosis in, midgestation may progress in utero to hypoplastic left heart, syndrome. In cases of critical aortic valvular stenosis, in utero, balloon valvuloplasty has been performed with good results.176, , Pulmonic Stenosis, Pulmonic stenosis may occur at the valve level or at the infundibulum. It occurs in 7.4% of live-born infants with CHD.46, Dysplastic and stenotic pulmonic valves are seen in Noonan, syndrome and with maternal rubella. Pulmonic stenosis is, associated with TAPVR, ASD, supravalvular aortic stenosis,, and TOF. The incidence of pulmonic stenosis is higher in, , monochorionic pregnancies and is 10 times more frequent in, the recipient twin of a pregnancy affected with twin-twin transfusion syndrome.177 In this setting the recipient’s heart becomes, hypertrophic secondary to increased preload. This is similar to, the ASH present in fetuses of diabetic mothers and results in, anatomic obstruction of the outflow tract.172, Increased velocity through the pulmonic valve and hypertrophy, of the right ventricle suggest pulmonic stenosis. Similar to aortic, stenosis, a real-time image of the stenotic pulmonic valve will, show it persisting in the field of view. As with aortic stenosis,, pulmonic stenosis tends to progress in utero. Pulmonic stenosis, has a variable outcome and can be managed with closed transventricular valvotomy or percutaneous balloon valvuloplasty.178,179, In utero pulmonary balloon valvuloplasty has also been successful, but is still in its early phase.180, , Cardiosplenic Syndromes, Cardiosplenic syndromes are syndromes associated with asplenia, (right isomerism) and polysplenia (left isomerism). Both are, defects of lateralization in which symmetrical development of, normally asymmetrical organs or organ systems occurs.180 Asplenia, (Ivemark syndrome) and polysplenia syndromes are usually, considered separate clinical entities. However, they have many, characteristics in common, including situs inversus or situs, ambiguus of various visceral organs, complex congenital heart, defects,181 and increased incidence of chronic arrhythmias.182, Pathologically, in asplenia or bilateral right-sidedness, left-sided, organs are a mirror image of normally right-sided organs. This, results in right atrial isomerism, bilateral trilobed lungs, bilateral, right bronchi and pulmonary arteries, ipsilateral location of the, aorta and IVC (either left or right side), absence of the spleen,, a midline horizontal liver, and bilateral SVC.183 In polysplenia, or bilateral left-sidedness, the right lung and bronchial tree, morphologically mirror those of the left. In many cases, intrahepatic interruption of the IVC with azygous continuation is, present, as are multiple spleens and left atrial isomerism.184, Cardiac anomalies associated with these syndromes include, TAPVR or PAPVR, ASD, VSD, univentricular heart, TGA, DORV,, and pulmonic/aortic stenosis or atresia. Coarctation, hypoplastic, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 24 : The Fetal Heart, , CHAPTER 37, , 1293, , Asplenia and Polysplenia:, Associated Findings, ASPLENIA, Absence of spleen, Bilateral right-sidedness, Right atrial isomerism, Bilateral trilobed lungs, Bilateral right bronchi and pulmonary arteries, Ipsilateral location of the aorta and IVC, Bilateral SVC, Situs ambiguous, Midline horizontal liver, POLYSPLENIA, Multiple spleens, Bilateral left-sidedness, Left atrial isomerism, Bilateral bilobed lungs, Bilateral left bronchi and pulmonary arteries, Interruption of IVC, Azygous continuation of IVC, Situs ambiguous, Midline horizontal liver, Congenital heart disease, Complete atrioventricular block, IVC, Inferior vena cava; SVC, superior vena cava., , left ventricle, mitral stenosis, cor triatriatum, dextrocardia, right, atrial hypoplasia, AVSD, truncus arteriosus, and TOF have also, been observed.184-188 Complete A-V block with an AVSD is, associated with polysplenia. Polysplenia syndrome is the second, most common disease associated with fetal heart block (after, L-TGA).186, Cardiosplenic syndromes should be considered when CHD, occurs with an arrhythmia. If these syndromes are suspected, a, careful search is made for the fetal spleen, which has been, visualized at 20 weeks’ gestation, with careful consideration of, the location of the fetal stomach.186,189 Other abnormal relationships, such as ipsilateral aorta and IVC (associated with asplenia), or interruption of the IVC with continuation of the azygous vein, (associated with polysplenia), may be documented prenatally., The mortality rate with cardiosplenic syndromes is extremely, high. Treatment depends largely on the type and number of, associated anomalies. Because cardiac malformations associated, with polysplenia are often less severe, they are more amenable, to surgical correction than lesions associated with asplenia.189,190, Neonates with asplenia have a higher mortality and postoperative, morbidity because of the high frequency of associated complex, cardiac malformations. In polysplenia the greatest attrition occurs, in the prenatal period and is often related to heart block with, resultant hydrops.188, , Cardiac Tumors, Fetal cardiac tumors are rare. Approximately 10% are malignant.191,192 Until the infant is 1 year of age, the majority of cardiac, , RA, , RV, , LA, LV, R, , FIG. 37.39 Rhabdomyoma. Apical four-chamber view shows an, echogenic mass in the left ventricle (LV), consistent with a rhabdomyoma, (R). LA, Left atrium; RA, right atrium; RV, right ventricle., , and pericardial masses are rhabdomyomas (58%) and teratomas, (19%). Cardiac fibromas account for approximately 12% of the, tumors in this age group. Other, less frequent tumors include, mesothelioma of the A-V node and cardiac hemangioma, (approximately 2% each)., Sonographically, fetal cardiac rhabdomyomas appear as solid,, echogenic masses. Rhabdomyomas (cardiac hamartomas) are, usually multiple, typically arising from the interventricular, septum193-199 (Fig. 37.39). Rhabdomyomas may develop in utero, after an initially normal fetal echocardiogram and may increase, in size and number over time.29,196,200-202 This finding underscores, the importance of serial fetal echocardiograms in fetuses at risk, for rhabdomyomas., Of patients with cardiac rhabdomyomas, 30% to 78% have, tuberous sclerosis.193-195 Other signs of tuberous sclerosis are, rarely found in fetal life, with the exception of subependymal, tubers in the brain, which can be detected with fetal MRI or by, mass effect leading to hydrocephalus.193 Unfortunately, the absence, of cardiac neoplasms in a fetus at risk for tuberous sclerosis does, not exclude this diagnosis.196, Cardiac tumors become hemodynamically significant by, causing obstruction to the outflow tracts or A-V valves, resulting, in congestive heart failure, hydrops, pericardial effusion, and, arrhythmias.193 Prognosis depends on the size, number,, and location of the tumor as well as associated arrhythmias and, anomalies. A meta-analysis of 138 published cases of fetal cardiac, rhabdomyomas found that size greater than 20 mm, presence, of arrhythmia, and hydrops were significantly associated with, increased morbidity.200 Infants with cardiac rhabdomyomas have, a guarded prognosis. Rhabdomyomas are hormone-sensitive, neoplasms, which explains their tendency to spontaneously, regress.203 Clinical manifestations associated with fetal cardiac, rhabdomyomas are diverse, ranging from spontaneous regression, of the tumor to sudden death.192 After birth, the cells lose the, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 25 : 1294, , Obstetric and Fetal Sonography, , PART IV, , ability to divide, rhabdomyomas can be expected to shrink or, completely resolve, and patients can often be treated conservatively.204 However, because of the association of rhabdomyomas, with tuberous sclerosis and arrhythmias, prognosis remains, guarded.205, Teratomas may appear as cystic, complex, or solid masses., These are intrapericardial in origin, with attachment to the aortic, root or pulmonary artery, and are virtually always associated, with a large pericardial effusion. Although teratomas are generally, considered to be benign, they can recur after surgical excision, and may undergo malignant degeneration.204 Fetal teratomas are, weakly associated with tuberous sclerosis.196, Rare cardiac tumors include fibromas and hemangiomas., Fibromas are almost always single and typically have central, necrosis and calcification.201 Fibromas are usually located in the, ventricular septum, making resection problematic. Hemangiomas, are even rarer and are usually associated with the right atrium, or interventricular septum, often with an intracavitary component., Cardiac hemangiomas are heterogeneous with cystic and solid, components, often with calcifications. They are frequently associated with a pericardial effusion.201, Echogenic foci within a ventricle should not be mistaken for, a cardiac tumor. These are thought to represent areas of mineralization of papillary muscle or chordae tendineae. These usually, occur in the left ventricle (93%) but may occur in the right, ventricle (5%) or both ventricles (2%) and are generally clinically, insignificant206-208 (Fig. 37.40). Echogenic foci have been associated, with chromosomal anomalies such as trisomy 21 and 13. However,, more recent literature suggests that in isolation, in an otherwise, low-risk pregnancy, an increased risk of aneuploidy does not, exist.208, Congenital ventricular aneurysms or diverticula may be, appreciated in utero. Both are usually isolated findings. Ventricular, aneurysms appear sonographically as an outpouching of the, ventricular myocardium that usually does not contract with the, ventricle. A diverticulum of the ventricle appears as a cystic, , RV, , RA, , LV, , LA, , FIG. 37.40 Echogenic Intracardiac Focus. Apical four-chamber view, of a fetal heart with an echogenic focus in the left ventricle (LV). LA,, Left atrium; RA, right atrium; RV, right ventricle., , structure connected to the ventricle by a narrow channel. These, often do contract with the associated ventricle during systole.209, , Cardiomyopathy, Cardiomyopathy encompasses a diverse group of cardiac disorders, with variable etiologies and anatomic and functional characteristics, and all result in an alteration in cardiac function. Cardiomyopathies account for 8% to 11% of fetal cardiovascular, disease,210 but only 1.8% of CHD in live-born infants.154 Approximately one-third of fetuses with cardiomyopathy die in utero,, which accounts for the difference in these numbers.211, A variety of conditions can cause fetal cardiomyopathy (Fig., 37.41), including viral and bacterial infections, inborn errors of, metabolism, endocardial fibroelastosis, familial cardiomyopathy, and maternal diabetes. Infectious agents act by damaging, the myocardium and producing a myocarditis with resultant, cardiomyopathy.212 In the setting of storage diseases, the myocardium becomes hypertrophic secondary to the accumulation, of various products within the myocardial cells. Many cases are, idiopathic.213, Endocardial fibroelastosis (EF) is a poorly understood process, in which diffuse endocardial thickening develops in one or more, cardiac chambers. EF is divided into two broad categories, primary, and secondary. Primary or isolated EF occurs in the absence of, other structural cardiac anomalies. In primary EF the affected, ventricle may be dilated or constricted.214 Viral myocarditis is, thought to be the cause of primary fetal EF, with mumps and, coxsackievirus B the most common infections.213,214 Secondary, EF occurs in the setting of a structural cardiac anomaly and, usually results in dilation of the affected chambers. The dilated, form occurs with coarctation of the aorta, aortic valvular, disease, mitral valvular disease, and other lesions. The less, common constricted form is associated with aortic stenosis or, atresia. In both types of EF the mural endocardium is largely, replaced by collagen and elastic tissue, giving it a glistening-white, appearance grossly213 and a striking echogenic appearance at, echocardiography (Fig. 37.42)., An increased incidence of asymmetrical septal hypertrophy, (ASH) or concentric hypertrophy has long been recognized, in infants of diabetic mothers.215 The characteristics of, these types of cardiomyopathy have been well documented, echocardiographically.215-218 Overall cardiac size in fetuses of, diabetic mothers can be increased as a result of the hypertrophy.219, Fetal hypertrophic cardiomyopathy can be expected in about, 20% of pregnancies with pregestational diabetes and appears to be, associated with an elevated third-trimester maternal hemoglobin, A1c and with an elevated neonatal insulinlike growth factor-1, (IGF-1) level.220 Regardless of the cause, severe cardiomyopathy, results in decreased cardiac function and a tendency to develop, congestive heart failure. This may be secondary to obstruction of, ventricular emptying in obstructive forms of cardiomyopathy or to, pump failure in nonobstructive forms. The sonographic diagnosis, of obstructive cardiomyopathy depends on the demonstration of, hypertrophy of the ventricular wall or septum. Nonobstructive, cardiomyopathy can be diagnosed by demonstrating dilation, of one or more cardiac chambers along with poor ventricular, contractility. About 37% of live-born infants with cardiomyopathy, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 26 : CHAPTER 37, , The Fetal Heart, , 1295, , P, LV, , LA, , RA, , S, SP, , RV, P, , LA, , RV, , RA, LV, , A, , B, , FIG. 37.41 Cardiomyopathy. (A) Concentric hypertrophy of the ventricular heart walls (P) and the interventricular septum (S) in fetus with, hypertrophic cardiomyopathy. (B) All four cardiac chambers are enlarged, occupying more than half the fetal chest in this fetus with dilated, cardiomyopathy. A pleural effusion is also seen. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; SP, spine., , RA, LA, , RV, LV, , FIG. 37.42 Endocardial Fibroelastosis. Subcostal four-chamber, view shows echogenic walls of the left ventricle (LV). LA, Left atrium;, RA, right atrium; RV, right ventricle., , 154, , have associated anomalies. The prognosis for fetal cardiomyopathy is variable, depending on the severity of the cardiac lesion,, the underlying cause, and associated anomalies. When hydrops, accompanies cardiomyopathy, the prognosis is poor., , Ectopia Cordis, Ectopia cordis is a rare malformation in which the heart is located, outside the thoracic cavity. It results from failure of fusion of, the lateral body fold in the thoracic region. Ectopia cordis is, classified by the following four types221:, , 1. Thoracic (60%). The heart is displaced from the thoracic, cavity through a sternal defect., 2. Abdominal (30%). The heart is displaced into the abdomen, through a diaphragmatic defect., 3. Thoracoabdominal (7%). The heart is displaced from the, chest through a defect in the lower sternum, with an associated, diaphragmatic or ventral abdominal wall defect (pentalogy, of Cantrell)., 4. Cervical (3%). The heart is displaced into the neck area., Although most cases are isolated, ectopia cordis may be, associated with pentalogy of Cantrell, a condition characterized, by a sternal cleft, ventral diaphragmatic hernia, omphalocele,, intracardiac anomalies, and ectopia cordis.222 A variety of cardiac, and chromosomal anomalies are associated with ectopia cordis., The sonographic diagnosis is generally not difficult and has been, made as early as 9 weeks’ gestation,223 although the abdominal, and cervical types have not been reported in utero (Fig. 37.43)., The prognosis is poor, with most infants dying within a few days, of birth.224, , ARRHYTHMIAS, Premature Atrial and, Ventricular Contractions, Premature atrial contractions (PACs) and premature ventricular, contractions (PVCs) are abnormal atrial or ventricular contractions originating from locations other than the sinus node. PACs, account for almost 75% and PVCs for 8% of fetal arrhythmias.40,225, PACs are associated with redundancy of the foraminal flap (atrial, septal aneurysm), maternal caffeine ingestion, and smoking.226,227, In 1% to 2% of PACs, a structural cardiac anomaly is present.37, PACs may be conducted or nonconducted. In either case,, the atrial pacemaker is “reset” so that the next normal atrial, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 27 : 1296, , PART IV, , Obstetric and Fetal Sonography, , beat is also early. PVCs have an early ventricular contraction, that is not preceded by an atrial contraction. The atrial pacemaker, is not reset, and the next normal beat occurs when expected, as, if the rhythm were regular. Thus PVCs are compensatory, allowing, the preexisting rhythm to continue, whereas PACs are generally, less than compensatory. PACs and PVCs may be distinguished, by M-mode or spectral Doppler sonography (see Fig. 37.15) by, placing the M-mode cursor or spectral Doppler sample volume, simultaneously through an atrial and ventricular structure. PACs, followed by ventricular contraction are described as “conducted,”, whereas a PAC that is not followed by a ventricular contraction, is “nonconducted” or “blocked.” These must be differentiated, , LV, RV, , LA, RA, SP, , FIG. 37.43 Thoracic Ectopia Cordis. The heart is located outside, the thorax. LA, Left atrium; LV, left ventricle; RA, right ventricle; RV,, right ventricle; SP, spine., , A, , from A-V block, in which a PAC does not occur. Conducted, PACs can be distinguished from PVCs by noting that a PAC, precedes the early ventricular beat and that, in the majority of, cases, PACs are less than compensatory whereas PVCs are generally compensatory., Premature contractions are benign arrhythmias in most, cases. Most disappear in utero or in the early neonatal period., From 1% to 2% of PACs may evolve into sustained, tachyarrhythmia.228, , Tachycardia, Fetal tachycardia is a heart rate greater than 180 beats/min., Supraventricular tachycardias (SVTs) are more common in the, fetus than ventricular tachycardias. Of cases of SVT, 5% to 10%, are associated with CHD.229, SVTs are classified as follows:, • Paroxysmal supraventricular tachycardia: atrial rate of 180, to 300 beats/min and a conduction rate of 1 : 1 (Fig. 37.44)., • Atrial flutter: atrial rate of 300 to 400 beats/min, frequently, associated with heart block, and a conduction rate of 2 : 1 to, 4 : 1, yielding a ventricular rate of 60 to 200 beats/min., • Atrial fibrillation: atrial rate of greater than 400 beats/min, and an irregular ventricular response at a rate of 120 to 160, beats/min., Ventricular tachycardia is defined as a rapid heart rate, associated with three or more consecutive premature ventricular, systoles., Fetal cardiac rhythm disturbances are usually first suspected on, the basis of auscultatory findings. M-mode and pulsed Doppler, echocardiography are useful in identifying and characterizing, tachycardias by observing the atrial and ventricular rates., The M-mode tracing is obtained through the heart to allow, independent assessment of atrial and ventricular wall motion., Further information is obtained from simultaneous recordings, of the atrial and ventricular walls or A-V and semilunar valve, motion. Spectral Doppler ultrasound evaluation can demonstrate, , B, , FIG. 37.44 Supraventricular Tachycardia. (A) M-mode tracing through the left ventricle and the right atrium shows a fetal heart rate of 273, beats/min and a 1 : 1 conduction. (B) Spectral Doppler ultrasound through the aortic valve in the same fetus shows a heart rate of 283 beats/min., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 28 : CHAPTER 37, , The Fetal Heart, , 1297, , HR-LV 60 bpm, , FIG. 37.45 Fetal Bradycardia. M-mode tracing shows ventricular, rate of 60 beats/min., , decreased flow velocities and cardiac output during SVT.230 Fetal, SVT can lead to fetal cardiovascular compromise, hydrops,, and death., Most fetal tachycardias have a relatively good prognosis and, can be treated in utero with various pharmacologic agents. Careful, interrogation of the tricuspid valve with color and spectral Doppler, ultrasound imaging is warranted in the setting of fetal tachycardia, because tricuspid valvular dysfunction with tricuspid regurgitation, can be the first indication of imminent congestive failure and, hydrops.231,232 In the presence of hydrops, immediate and aggressive therapy is warranted.225,228,233-238 Several antiarrhythmic drugs, are available to attempt to cardiovert SVTs in utero. Prognosis, in the setting of fetal tachyarrhythmia depends on a number of, factors, including type and duration of arrhythmia, presence of, structural cardiac anomalies, gestational age, and response to, intrauterine therapy.231, , Bradycardia, Fetal bradycardia is a “prolonged” heart rate of 100 beats/min, or less (Fig. 37.45). Approximately 5% of fetal arrhythmias are, classified as bradycardia.37 Transient bradycardia can be related, to an increase in intrauterine pressure, occasionally secondary, to the pressure of an ultrasound transducer or fetal compression, of the umbilical cord.239,240 This is often seen during fetal sonograms and is of no clinical consequence, unless the bradyarrhythmia is sustained., Sinus bradycardia without fetal hypoxia is rare.240 If less than, 80 beats/min, sinus bradycardia may be associated with fetal, asphyxia. A fetal heart rate below 80 beats/min lasting longer, than 13 minutes is associated with acidemia-induced cerebral, palsy.241 Heart rates lower than 100 beats/min during the first, trimester are associated with an increased risk of fetal demise.242, When bradycardia is associated with an increased nuchal, translucency, risk for CHD is increased, and early transvaginal, fetal echocardiography should be considered. Complex CHD, has been detected in the first trimester in this clinical setting.242, The fetus with sustained bradycardia should have careful follow-up, and monitoring for signs of cardiac failure. Persistent bradycardia, may warrant early delivery. In utero treatment has had limited, success.243, , FIG. 37.46 Complete Heart Block. M-mode tracing shows a slow, ventricular rate (V) of 47 beats/min, with an irregular disassociated atrial, rate (A)., , Congenital Heart Block, Failure of transmission of impulses from the atrium to the, ventricles results in atrioventricular block (AVB). AVB is classified into the following three types:, • First-degree block: a prolonged PR interval results in a, conduction delay, but without markedly abnormal rate or, rhythm. This may only be appreciated in utero by measuring, the PR interval on spectral Doppler tracing., • Second-degree block: with blockage of a single atrial beat, (Mobitz type I) or with intermittent conduction abnormalities,, such that the ventricular rate is a fraction of the atrial rate, (Mobitz type II)., • Third-degree or complete heart block: the ventricular and, atrial rates are entirely dissociated (Fig. 37.46)., In the normal heart, an electrical impulse originates from the, sinoatrial (SA) node and travels to the A-V node and through, the Purkinje system to the ventricles. AVB results from immaturity or complete absence of the conducting system or from, abnormalities at the level of the A-V node. This rare disorder is, associated with maternal collagen vascular disease (systemic, lupus erythematosus).244 After one affected child, further, pregnancies carry a 17% to 30% risk for AVB.245,246 Serial fetal, echocardiograms are useful in the fetus at risk for congenital, complete heart block related to maternal lupus. This monitoring, allows early detection and treatment of the immune-mediated, fetal myocarditis and congenital complete heart block.247-249, In the absence of associated structural abnormalities, the, prognosis for a fetus with congenital heart block is good. However,, at least 40% of fetuses with complete heart block have structural, cardiac abnormalities.37 When associated cardiac anomalies exist,, the prognosis is poor. Fetuses with AVB are at risk for heart, failure and should be monitored closely. Overall, the vast majority, of fetuses with arrhythmias have a good outcome. The incidence, of associated structural cardiac disease is as low as 0.3%, similar, to that in the general population, and the incidence of significant, arrhythmia is only 1.6%.249, , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 29 : 1298, , PART IV, , Obstetric and Fetal Sonography, , REFERENCES, 1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll, Cardiol. 2002;39(12):1890-1900., 2. Tegnander E, Williams W, Johansen OJ, et al. Prenatal detection of heart, defects in a non-selected population of 30,149 fetuses—detection rates and, outcome. Ultrasound Obstet Gynecol. 2006;27(3):252-265., 3. Young ID, Clarke M. Lethal malformations and perinatal mortality: a 10, year review with comparison of ethnic differences. Br Med J (Clin Res Ed)., 1987;295(6590):89-91., 4. Jones KL, Smith DW. Smith’s recognizable patterns of human malformation., 6th ed. Philadelphia: Saunders-Elsevier; 2006., 5. Nora JJ. Medical genetics: principles and practice. 4th ed. Philadelphia: Lea, & Febiger; 1994., 6. Nyberg DA, Mahony BS, Pretorius D. Diagnostic ultrasound of fetal, anomalies: text and atlas. Chicago: Year Book Medical Publishers; 1990., 7. Romero R. Prenatal diagnosis of congenital anomalies. Norwalk, CT: Appleton, & Lange; 1988., 8. Nora JJ, Nora AH. Maternal transmission of congenital heart diseases: new, recurrence risk figures and the questions of cytoplasmic inheritance and, vulnerability to teratogens. Am J Cardiol. 1987;59(5):459-463., 9. Reed KL. Fetal echocardiography. Semin Ultrasound CT MR. 1991;, 12(1):2-10., 10. Greenwood RD, Rosenthal A, Parisi L, et al. Extracardiac abnormalities, in infants with congenital heart disease. Pediatrics. 1975;55(4):485492., 11. Berg KA, Boughman JA, Astemborski JA, et al. Implications for prenatal, cytogenetic analysis from Baltimore-Washington study of liveborn infants, with confirmed congenital heart defects (CHD). Am J Hum Genet., 1986;39:A50., 12. Berg KA, Clark EB, Astemborski JA, Boughman JA. Prenatal detection of, cardiovascular malformations by echocardiography: an indication for, cytogenetic evaluation. Am J Obstet Gynecol. 1988;159(2):477-481., 13. Wladimiroff JW, Stewart PA, Sachs ES, Niermeijer MF. Prenatal diagnosis, and management of congenital heart defect: significance of associated fetal, anomalies and prenatal chromosome studies. Am J Med Genet. 1985;21(2):, 285-290., 14. Allan LD. Fetal echocardiography. Clin Obstet Gynecol. 1988;31(1):, 61-79., 15. Callan NA, Maggio M, Steger S, Kan JS. Fetal echocardiography: indications, for referral, prenatal diagnoses, and outcomes. Am J Perinatol. 1991;8(6):, 390-394., 16. Bromley B, Estroff JA, Sanders SP, et al. Fetal echocardiography: accuracy, and limitations in a population at high and low risk for heart defects. Am, J Obstet Gynecol. 1992;166(5):1473-1481., 17. Copel JA, Kleinman CS. The impact of fetal echocardiography on perinatal, outcome. Ultrasound Med Biol. 1986;12(4):327-335., 18. Simpson LL. Indications for fetal echocardiography from a tertiary-care, obstetric sonography practice. J Clin Ultrasound. 2004;32(3):123128., 19. Bahtiyar MO, Dulay AT, Weeks BP, et al. Prevalence of congenital heart, defects in monochorionic/diamniotic twin gestations: a systematic literature, review. J Ultrasound Med. 2007;26(11):1491-1498., 20. Bahtiyar MO, Campbell K, Dulay AT, et al. Is the rate of congenital heart, defects detected by fetal echocardiography among pregnancies conceived, by in vitro fertilization really increased? A case-historical control study., J Ultrasound Med. 2010;29(6):917-922., 21. Vergani P, Mariani S, Ghidini A, et al. Screening for congenital heart disease, with the four-chamber view of the fetal heart. Am J Obstet Gynecol., 1992;167(4 Pt 1):1000-1003., 22. Copel JA, Pilu G, Green J, et al. Fetal echocardiographic screening for, congenital heart disease: the importance of the four-chamber view. Am J, Obstet Gynecol. 1987;157(3):648-655., 23. Kirk JS, Riggs TW, Comstock CH, et al. The prospective evaluation of fetal, cardiac anatomy: a comparison of 4 and 5 chamber views in 5967 patients., Am J Obstet Gynecol. 1993;168:291., 24. Wyllie J, Wren C, Hunter S. Screening for fetal cardiac malformations. Br, Heart J. 1994;71(4 Suppl.):20-27., , 25. Sharland GK, Allan LD. Screening for congenital heart disease prenatally., Results of a 2 1/2-year study in the South East Thames Region. Br J Obstet, Gynaecol. 1992;99(3):220-225., 26. Tegnander E, Eik-Nes SH, Johansen OJ, Linker DT. Prenatal detection of, heart defects at the routine fetal examination at 18 weeks in a non-selected, population. Ultrasound Obstet Gynecol. 1995;5(6):372-380., 27. Comstock CH. Normal fetal heart axis and position. Obstet Gynecol., 1987;70(2):255-259., 28. AIUM practice guideline for the performance of fetal echocardiography., J Ultrasound Med. 2013;32(6):1067–1082., 29. Yagel S, Weissman A, Rotstein Z, et al. Congenital heart defects: natural, course and in utero development. Circulation. 1997;96(2):550-555., 30. Trines J, Hornberger LK. Evolution of heart disease in utero. Pediatr Cardiol., 2004;25(3):287-298., 31. Simpson JM, Sharland GK. Fetal tachycardias: management and outcome, of 127 consecutive cases. Heart. 1998;79(6):576-581., 32. Dolkart LA, Reimers FT. Transvaginal fetal echocardiography in early, pregnancy: normative data. Am J Obstet Gynecol. 1991;165(3):688691., 33. Achiron R, Weissman A, Rotstein Z, et al. Transvaginal echocardiographic, examination of the fetal heart between 13 and 15 weeks’ gestation in a, low-risk population. J Ultrasound Med. 1994;13(10):783-789., 34. Weiner Z, Lorber A, Shalev E. Diagnosis of congenital cardiac defects between, 11 and 14 weeks’ gestation in high-risk patients. J Ultrasound Med., 2002;21(1):23-29., 35. Cartier MS, Davidoff A, Warneke LA, et al. The normal diameter of the, fetal aorta and pulmonary artery: echocardiographic evaluation in utero., Am J Roentgenol. 1987;149(5):1003-1007., 36. Yagel S, Cohen SM, Achiron R. Examination of the fetal heart by five, short-axis views: a proposed screening method for comprehensive cardiac, evaluation. Ultrasound Obstet Gynecol. 2001;17(5):367-369., 37. Reed KL. Fetal arrhythmias: etiology, diagnosis, pathophysiology, and, treatment. Semin Perinatol. 1989;13(4):294-304., 38. Gembruch U, Chatterjee MS, Bald R, et al. Color Doppler flow mapping, of fetal heart. J Perinat Med. 1991;19(1-2):27-32., 39. Chiba Y, Kanzaki T, Kobayashi H, et al. Evaluation of fetal structural heart, disease using color flow mapping. Ultrasound Med Biol. 1990;16(3):, 221-229., 40. Benacerraf BR, Sanders SP. Fetal echocardiography. Radiol Clin North Am., 1990;28(1):131-147., 41. Sharland GK, Chita SK, Allan LD. The use of colour Doppler in fetal, echocardiography. Int J Cardiol. 1990;28(2):229-236., 42. Yagel S, Cohen SM, Shapiro I, Valsky DV. 3D and 4D ultrasound in fetal, cardiac scanning: a new look at the fetal heart. Ultrasound Obstet Gynecol., 2007;29(1):81-95., 43. Acar P, Dulac Y, Taktak A, Abadir S. Real-time three-dimensional fetal, echocardiography using matrix probe. Prenat Diagn. 2005;25(5):370375., 44. Brekke S, Tegnander E, Torp HG, Eik-Nes SH. Tissue Doppler gated (TDOG), dynamic three-dimensional ultrasound imaging of the fetal heart. Ultrasound, Obstet Gynecol. 2004;24(2):192-198., 45. Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment, of fetal cardiac disease: a scientific statement from the American Heart, Association. Circulation. 2014;129(21):2183-2242., 46. Hoffman JI, Christianson R. Congenital heart disease in a cohort of, 19,502 births with long-term follow-up. Am J Cardiol. 1978;42(4):, 641-647., 47. Allan LD, Crawford DC, Anderson RH, Tynan M. Spectrum of congenital, heart disease detected echocardiographically in prenatal life. Br Heart J., 1985;54(5):523-526., 48. Keith JD. Atrial septal defect: ostium secundum, ostium primum, and, atrioventricularis communis (common AV canal). In: Keith JD, Rowe RD,, Vlad P, editors. Heart disease in infancy and childhood. 3rd ed. New York:, Macmillan; 1978. p. 330-404., 49. Samanek M. Children with congenital heart disease: probability of natural, survival. Pediatr Cardiol. 1992;13(3):152-158., 50. Fyler DC. Atrial septal defect secundum. In: Fyler DC, editor. Nadas’ pediatric, cardiology. Philadelphia: Hanley and Belfus; 1992. p. 513-524., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.
Page 30 : CHAPTER 37, 51. Feldt RH, Avasthey P, Yoshimasu F, et al. Incidence of congenital heart, disease in children born to residents of Olmsted County, Minnesota, 19501969. Mayo Clin Proc. 1971;46(12):794-799., 52. Crelin ES. Anatomy of the newborn; an atlas. Philadelphia: Lea & Febiger;, 1969., 53. Kachalia P, Bowie JD, Adams DB, Carroll BA. In utero sonographic appearance, of the atrial septum primum and septum secundum. J Ultrasound Med., 1991;10(8):423-426., 54. Wilson AD, Rao PS, Aeschlimann S. Normal fetal foramen flap and transatrial, Doppler velocity pattern. J Am Soc Echocardiogr. 1990;3(6):491-494., 55. Benacerraf BR, Pober BR, Sanders SP. Accuracy of fetal echocardiography., Radiology. 1987;165(3):847-849., 56. DeVore GR, Horenstein J, Siassi B, Platt LD. Fetal echocardiography. VII., Doppler color flow mapping: a new technique for the diagnosis of congenital, heart disease. Am J Obstet Gynecol. 1987;156(5):1054-1064., 57. Cockerham JT, Martin TC, Gutierrez FR, et al. Spontaneous closure of, secundum atrial septal defect in infants and young children. Am J Cardiol., 1983;52(10):1267-1271., 58. Steele PM, Fuster V, Cohen M, et al. Isolated atrial septal defect with, pulmonary vascular obstructive disease—long-term follow-up and prediction, of outcome after surgical correction. Circulation. 1987;76(5):10371042., 59. Ferencz C, Rubin JD, McCarter RJ, et al. Cardiac and noncardiac malformations: observations in a population-based study. Teratology. 1987;35(3):, 367-378., 60. Goor DA, Lillehei CW. Congenital malformations of the heart: embryology,, anatomy, and operative considerations. New York: Grune & Stratton;, 1975., 61. Grahm TP, Bender HW, Spach M. Ventricular septal defects. In: Moss AJ,, Adams FH, Emmanouilides GC, editors. Heart disease in infancy, childhood,, and adolescence. 2nd ed. Baltimore: Williams & Wilkins; 1977., 62. Allan LD, Crawford DC, Anderson RH, Tynan MJ. Echocardiographic and, anatomical correlations in fetal congenital heart disease. Br Heart J., 1984;52(5):542-548., 63. Allan LD, Tynan M, Campbell S, Anderson RH. Identification of congenital, cardiac malformations by echocardiography in midtrimester fetus. Br Heart, J. 1981;46(4):358-362., 64. Parness IA, Yeager SB, Sanders SP, et al. Echocardiographic diagnosis of, fetal heart defects in mid trimester. Arch Dis Child. 1988;63(10 Spec, No):1137-1145., 65. Yagel S, Sherer D, Hurwitz A. Significance of ultrasonic prenatal diagnosis, of ventricular septal defect. J Clin Ultrasound. 1985;13(8):588-590., 66. Brown DL, Emerson DS, Cartier MS, et al. Congenital cardiac anomalies:, prenatal sonographic diagnosis. Am J Roentgenol. 1989;153(1):109114., 67. Sutherland GR, Smyllie JH, Ogilvie BC, Keeton BR. Colour flow imaging, in the diagnosis of multiple ventricular septal defects. Br Heart J., 1989;62(1):43-49., 68. Garne E. Atrial and ventricular septal defects—epidemiology and spontaneous, closure. J Matern Fetal Neonatal Med. 2006;19(5):271-276., 69. Hoffman JI. Natural history of congenital heart disease. Problems in its, assessment with special reference to ventricular septal defects. Circulation., 1968;37(1):97-125., 70. Hoffman JI, Rudolph AM. The natural history of isolated ventricular septal, defect with special reference to selection of patients for surgery. Adv Pediatr., 1970;17:57-79., 71. Hoffman JI, Rudolph AM. The natural history of ventricular septal defects, in infancy. Am J Cardiol. 1965;16(5):634-653., 72. Crawford DC, Chita SK, Allan LD. Prenatal detection of congenital heart, disease: factors affecting obstetric management and survival. Am J Obstet, Gynecol. 1988;159(2):352-356., 73. Machado MV, Crawford DC, Anderson RH, Allan LD. Atrioventricular, septal defect in prenatal life. Br Heart J. 1988;59(3):352-355., 74. Carvalho JS, Rigby ML, Shinebourne EA, Anderson RH. Cross sectional, echocardiography for recognition of ventricular topology in atrioventricular, septal defect. Br Heart J. 1989;61(3):285-288., 75. Freedom RM, Culham JAG, Moes CAF. Angiocardiography of congenital, heart disease. New York: Macmillan; 1984., , The Fetal Heart, , 1299, , 76. Becker AE, Anderson RH. Atrioventricular septal defects: What’s in a name?, J Thorac Cardiovasc Surg. 1982;83(3):461-469., 77. Fontana RS, Edwards JE. Congenital cardiac disease. Philadelphia: Saunders;, 1962., 78. Shenker L, Reed KL, Marx GR, et al. Fetal cardiac Doppler flow studies, in prenatal diagnosis of heart disease. Am J Obstet Gynecol. 1988;158, (6 Pt 1):1267-1273., 79. Gembruch U, Knopfle G, Chatterjee M, et al. Prenatal diagnosis of atrioventricular canal malformations with up-to-date echocardiographic technology: report of 14 cases. Am Heart J. 1991;121(5):1489-1497., 80. Rasiah SV, Ewer AK, Miller P, et al. Outcome following prenatal diagnosis, of complete atrioventricular septal defect. Prenat Diagn. 2008;28(2):, 95-101., 81. Allan LD, Crawford DC, Sheridan R, Chapman MG. Aetiology of nonimmune hydrops: the value of echocardiography. Br J Obstet Gynaecol., 1986;93(3):223-225., 82. Bader RS, Punn R, Silverman NH. Evaluation of risk factors for prediction, of outcome in fetal spectrum of atrioventricular septal defects. Congenit, Heart Dis. 2014;9(4):286-293., 83. Bakhtiary F, Takacs J, Cho MY, et al. Long-term results after repair of, complete atrioventricular septal defect with two-patch technique. Ann, Thorac Surg. 2010;89(4):1239-1243., 84. Genton E, Blount Jr SG. The spectrum of Ebstein’s anomaly. Am Heart J., 1967;73(3):395-425., 85. Lev M, Liberthson RR, Joseph RH, et al. The pathologic anatomy of Ebstein’s, disease. Arch Pathol. 1970;90(4):334-343., 86. Roberson DA, Silverman NH. Ebstein’s anomaly: echocardiographic and, clinical features in the fetus and neonate. J Am Coll Cardiol. 1989;14(5):, 1300-1307., 87. Zuberbuhler JR, Becker AE, Anderson RH, Lenox CC. Ebstein’s malformation, and the embryological development of the tricuspid valve. With a note on, the nature of “clefts” in the atrioventricular valves. Pediatr Cardiol. 1984;, 5(4):289-295., 88. Anderson KR, Zuberbuhler JR, Anderson RH, et al. Morphologic spectrum, of Ebstein’s anomaly of the heart: a review. Mayo Clin Proc. 1979;54(3):, 174-180., 89. Stewart PA, Wladimiroff JW, Reuss A, Sachs ES. Fetal echocardiography:, a review of six years experience. Fetal Ther. 1987;2(4):222-231., 90. Sharland GK, Chita SK, Allan LD. Tricuspid valve dysplasia or displacement, in intrauterine life. J Am Coll Cardiol. 1991;17(4):944-949., 91. Nora JJ, Nora AH, Toews WH. Letter: Lithium, Ebstein’s anomaly, and, other congenital heart defects. Lancet. 1974;2(7880):594-595., 92. Nora JJ, Nora AH. The evolution of specific genetic and environmental, counseling in congenital heart diseases. Circulation. 1978;57(2):205213., 93. Schou M, Goldfield MD, Weinstein MR, Villeneuve A. Lithium and pregnancy., I. Report from the Register of Lithium Babies. Br Med J. 1973;2(5859):, 135-136., 94. Weinstein MR, Goldfield M. Cardiovascular malformations with lithium, use during pregnancy. Am J Psychiatry. 1975;132(5):529-531., 95. Park JM, Sridaromont S, Ledbetter EO, Terry WM. Ebstein’s anomaly of, the tricuspid valve associated with prenatal exposure to lithium carbonate., Am J Dis Child. 1980;134(7):703-704., 96. Kallen B, Tandberg A. Lithium and pregnancy. A cohort study on manicdepressive women. Acta Psychiatr Scand. 1983;68(2):134-139., 97. Jacobson SJ, Jones K, Johnson K, et al. Prospective multicentre study of, pregnancy outcome after lithium exposure during first trimester. Lancet., 1992;339(8792):530-533., 98. Anderson RH, Silverman NH, Zuberbuhler JR. Congenitally unguarded, tricuspid orifice: its differentiation from Ebstein’s malformation in association, with pulmonary atresia and intact ventricular septum. Pediatr Cardiol., 1990;11(2):86-90., 99. Oberhoffer R, Cook AC, Lang D, et al. Correlation between echocardiographic, and morphological investigations of lesions of the tricuspid valve diagnosed, during fetal life. Br Heart J. 1992;68(6):580-585., 100. Handler JB, Berger TJ, Miller RH, et al. Partial atrioventricular canal in, association with Ebstein’s anomaly. Echocardiographic diagnosis and surgical, correction. Chest. 1981;80(4):515-517., , Downloaded for Abhishek Srivastava (
[email protected]) at Fortis Escorts Heart Institute and Research Centre from ClinicalKey.com by, Elsevier on January 07, 2022. For personal use only. No other uses without permission. Copyright ©2022. Elsevier Inc. All rights reserved.