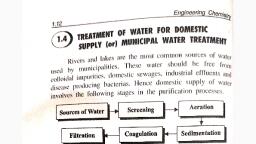
Page 1 :
t.2a7, , its Treatment, , and!, V7 TREATMENT OF BOILER FEED WATER, ; (SOFTENING or CONDITIONING METHODS), , water, , er used for industrial purposes should be free from, substances, suspended impurities and, gissolved gases ele. The process of removing hardmess, , salts from water IS known as softening (or), , oducing, conditioning of water., , Wal, hardness producing, , Softening of water can be done in two methods, , i. Internal treatment., 2, External treatment., , 1.7.1 Internal Conditioning (or) Internal, Treatment (or) Boiler Compounds, , It involves the removal of scale forming substance. which, were not completely removed in the external treatment, by, adding chemicals directly into the boiler. This chemicals are, also called boiler compounds., , 1. Phosphate conditioning, , Scale formation can be, phosphate. It is used in high pressure, , . 2 2 . =, reacts with Ca? *andMg~ salts to give soft sludges of, calcium and magnesium phosphates., , avoided by adding sodium, boilers. The phosphate, , 3 CaSO, +2 Na3PO4 —_ Ca3(PO,)> * 3Na,SO4, , Generally 3 types of phosphates are employed,, , (a) Trisodium phosphate - Na3PO4 (Too alkaline) - used for, , too acidic water., ° Disodium hydrogen phosphate, alkaline) - used for weakly acidic water., NaHPO4 (acidic) ~ used, , - Na HPO, (weakly, , = Sodium dihydrogen phosphate Or alkaline water.
Page 2 :
Engineerin Chemist, 1.28, , 2, Colloidal conditioning << enilleial, Scale formation can be avoided by adding, , ; in, etc., It is, conditioning agents like kerosene, agen Be tances get, used in low pressure boilers. These colloidal § then it, coated over the scale forming particles and conver i ecaite, non-adherent, loose precipitate called sludge, whic, , removed by blow down operation., , 3. Sodium aluminate conditioning, Sodium aluminate (NaAIO,) under goes hydrolysis in, , boiler water to give gelatinous white precipitate of aluminium, hydroxide and sodium hydroxide., , NaAlO, + 2H,0 ——>Al(OH)3 + NaOH, , The sodium hydroxide, thus formed, precipitates, magnesium as magnesium hydroxide. The gelatinous, precipitates of aluminium hydroxide and magnesium hydroxide, entrap the colloidal silica and finely divided solids and settled, easily. This can be removed easily by blow down operations., , 4. Calgon conditioning, Calgon is sodium = hexa’_—s meta_— phosphate, Nay [Nag(PO3)¢]. This substance interacts with calcium ions, , forming a highly soluble complex and thus prevents the, precipitation of scale forming salt., , 2 CaSO4 + Nag[Na4(PO3)¢] ——> Nag[Cap(PO3)¢] +2 Na SOj4., , The complex Naz [Ca,(PO})¢] is soluble in water and, , there is no problem of sludge disposal. So calgon conditioning, is better than phosphate conditioning.
Page 3 :
wator and ite Treatment 439, , 1.7.2 External conditioning (or) External, Treatment, , Ir involves the removal of hardness producing salts from, the water before feeding into the boiler, The external treatment, can be done by two process, , 1, Demineralisation (or) Jon-exchange process, 2. Zeolite process., , 1. Jon Exchange (or) Demineralisation process, This process removes almost all the ions (both anions, and cations) present in hard water., , The soft water, produced by zeolite processes, does not, contain hardness producing Ca* andMg** ions, but it contains, other ions like Na*, Kt, SO7”, CI etc., On the other hand D.M., (Demineralised) water does not contain both anions and, cations., , Thus a soft water is not demineralised water whereas a, demineralised water is soft water., , Demineralisation process is carried out by using ion, exchange resins, which are long chain, cross linked, insoluble, organic polymers with a microporous structure. The functional, , groups attached to the chains. are responsible for the ion, exchanging properties. The following two types of ion, , exchange resins are used., 1. Cation exchange resin (or) cation exchanger, , 2. Anion exchange resin (or) Anion exchanger, , I. Cation exchanger, Resins containing acidic fonation ee, , + i :, (Coon, -SO4H) are capable of exchanging their H ;, other cations of hard water. Cation exchange resin, , "presented as RH,, , ions with, is
Page 4 :
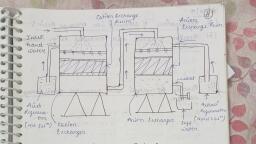
1.30 Engineering Chemistry, , Examples oa, , (i) Sulphonated coals., , , , (ii) Sulphonated polystyrene., , R-SO3H; R-COOH = RH,, , 2. Anion Exchanger, , Resins containing basic functional groups (-NH, -OH), are capable of exchanging their anions with other anions of, hard water. Anion exchange resin is represented as R (OH))., , Examples >, , (i) Cross-linked quaternary ammonium salts., , (il) Urea-formaldehyde resin., , , , R—-NR3OH ; R-OH; R-NH)=R(OH),, , Process, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , Alkali for, ‘Anion regeneration, , , , , , , , , , Tegeneration t, , , , , , , , , , , , , , , , , , , , , , , , alls halls. Deionised water, Wash —>, , Fig. 1.6 Demineralisation process
Page 5 :
its Treatment, fer and _its, wa 1.31, , The hard water first passed through a cation exch ung, f xchange, , column, (Fig. 1.6) which absorbs all the cations — like, , ca’, Mg, Na‘, K*, etc., present in the hard water,, RH) + CaCly —->RCa + 2HC], RH, + MgSO, -—>RMg+ H,SO,, RH + NaCl —>RNa + HCI, , The cation free water is then passed through a anion, exchange column, which absorbs all the anions like, , cr, $Or HCO;, etc., present in the water., R’(OH), + 2HCl —>R’ Ch, + 2H,0, R’(OH), + H)SO, —>R’SO, + 2H,0, , The water coming out of the anion exchanger is, completely free from cations and anions. This water is known, as demineralised water (or) deionised water., , Regeneration, When the cation exchange resin is exhausted, it can be, regenerated by passing a solution of dil HCl (or) dil HySOy., , RCa + 2HCI —->RH) + CaCl,, , RNa + HCl —>RH + NaCl, , . Similarly, when the anion exchange resin is exhausted,, can be regenerated by passing a solution of dil NaOH., , R’Cl, + 2NaOH—>R(OH)2 + 2NaCl., Advantages of ion-exchange process, , (i) Highly acidic (or) alkaline water ca, by this process., , n be treated