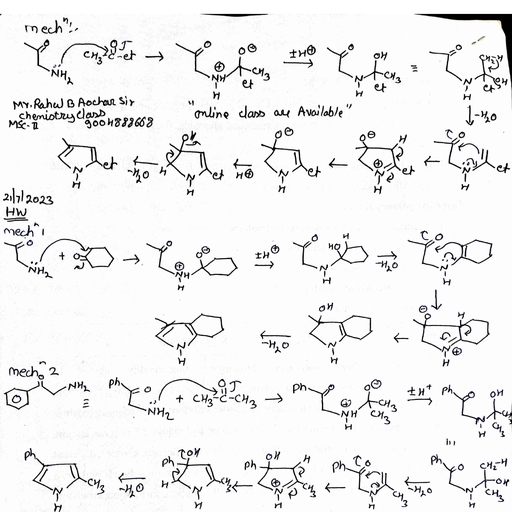
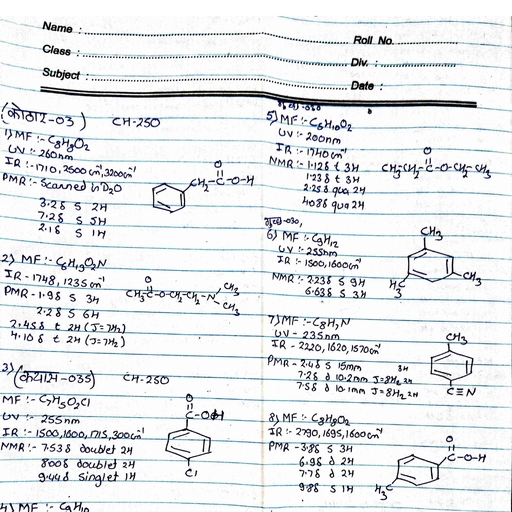
Page 1 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , Dr. R.V.PATIL (HOD), , Chapter, No. 1, , Mr.R.B. Aochar, , STRUCTURAL ISOMERISM, Staggered, , Classification, isomersim :, , Eclipsed, , Stereoisomerism, , of, , strucural, , 1. Chain or nuclear isomerism :, CH3, , Introduction :, Stereochemistry is the study of the, “three-dimensional” structure of molecules. The, foundation of organic stereochemistry was laid by, Vant’s hoff and Le Bel in 1874. They proposed that, the four bond to carbon were directed towards the, corners of a tetrahedron. Stereochemistry, explain, the existence of stereochemical, isomers or, stereoisomers namely Conformational ( those that, can be interconverted by rotation about a sigma, bond) and Configurational (those that can be, interconverted only by breaking and reforming of, bonds ). Two important sub-classes of, configurational isomers are geometrical isomers, (those in which restricted rotation in a ring or at a, multiple bond determine the relative spatial, arrangement of atoms ) and optical isomers ( those, that differ in the three dimensional relationship of, substituent’s about one or more atoms)., , CH3CHCH2CH3, , pentane, , isopentane, , CH3, neopentane, , Compounds which have the same molecular, formula but different structure (straight or, branched) of the carbon chain are called as chain, isomers and the phenomenon is called chain, isomerism. eg, Butane (C5H12) has three isomers., , 2. Poistion isomerism :, , OH, , OH, , Compounds which have, the same structure of, CH3CHCH3, CH3CH2CH2, carbon chain but differ, propan-2-ol, only in the position of propan-1-ol, the multiple (double or triple) bond or, thefunctional group are called position isomers and, the phenomenon is called position isomerism., , 3., , Functional isomerism : Compounds, , C2H6O, represents, , ISOMERISM, , STEREOISOMERISM, Imp., , Chain, Position, , CH3CCH3, , CH3CH2CH2CH2CH3, , having the same molecular formula but different, functional group are called functional isomers and, the phenomenon is called functional isomerism., For example,, , Type of isomerism :, , STRUCTURAL, , CH3, , Conformational, , Configurational, , 4., , CH3CH2OH, , CH3OCH3, , ethanol, , methoxymethane, , Metamerism isomerism : Compounds, , having the same molecular formula but diffents, number of carbon atoms or differents alkyl group, on either side of the functional group (i.e., -O,-S, and –NH-) are metamers and the phenomenon is, called metamerism. For example,, , Functional, Metamerism, , geometrical, isomers, , Optical, isomers, , Tautomerism, Ring chain, , Cis, , Enantiomers, , Trans, , Diastereomers, , CH3OCH2CH2CH3, , CH3CH2OCH2CH3, , 1-methoxypropane, , 2-ethoxyethane, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 1
Page 2 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , 5. Tautomersim isomerism : It is areies due, to 1,3 migration of a protron from one, polyvalent atom to the other within the same, molecules. The two isomers thus obtained, which exit in dynamic equilibrium with each, other are called tautomers and the phenomenon, is called tautomersim. For example,, O, , H3C, , OH, H, , ethenol, , 6. Ring Chain isomerism : Compunds having, the same molecular fromula but the possessing, cyclic and open chain structure are called ring, chain isomers and the phenomenon is called as, ring chain isomersism. For example,, , C3H6, represents, , CH3CH=CH2, prop-1-ene, , Mr.R.B. Aochar, , Vairious methods have been develpoed for the two, dimensional representation of three dimensional, structures.These are :, (i), (ii), (iii), (iv), , Flying –wedge representation, Fisher-projection formula, Sawhorse formula, Newman formula, , H, , H2C, , acetaldehyde, , Dr. R.V.PATIL (HOD), , and, cyclopropane, , 1. The Flying-wedge Representation, This is the most commonly used representation. In, this representation three types of lines are used in a, standard way to indicates three dimensional, structures in two dimensional picture. A solid, wedge, (, ) (thick line) represent a bond, projecting above the plane of the paper (towards, observer). Continuous lines (, ) (solid, lines) are bonds in the plane of the paper. A broken, wedge (, ) (dashed line) is a bond below the, plane of the paper. i.e. bond pointing away from the, observer., Below the palne, of the paper, , STEREOISOMERISM, , CHO, , In the plane, of the paper, , Isomerism: The compounds having, the same molecular formula but, different, physical, and, chemical, properties are called isomers (Greek : isos = equal,, meros = part) and this phenomenon is known as, isomerism. Eg., C5H12 same molecular formula but, different structural., , Stereoisomerism: Isomers having, the same constitution but different, spatial arrangements of their atoms or, groups are known as stereoisomers and, the isomerism exhibited by them is called, stereoisomerism., There are two types of, stereoisomerism: configurational isomerism and, conformational isomerism. The different spatial, arrangements of atoms in a molecule which are, redialy interconvertible by rotation about single, bonds are called conformations., , H, OH, HOH2C, Above the plane, of the paper, , 2. Fisher Projection Formula, This is also called fisher projection in which all, bonds are drawn as solid lines with the, understanding that horizontal bonds point towards, observer (below the plane of the paper) and vertical, bonds point away from the observer (below the, plane of the paper). The chiral carbon lines in the, plane of the paper and is usually omitted, (structure II)., CHO, , H, , C, , CHO, , OH, , CH2OH, , PROJECTION FORMULA, , (I), , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , H, , OH, , CH2OH, , (II), , Page 2
Page 3 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , The conformation which is drawn in a Fisher, projection formula of any compound containing, more than one stereocentre happens to be least, stable, fully eclipsed conformation of the molecule., , 3. Sawhorse Formula, The Sawhorse formula indicates the spatial, arrangements of all the atoms or groups on two, adjacent carbon atoms. The bond between the two, adjacent carbon atoms is represented by a diagonal, line usually from lower left to upper right. The left, hand bottom end representing the atom nearest to, the observer and the right hand top end representing, the atom further away to the observer. Two of the, remaining bonds to the two carbons are drawn, vertically and three other four bonds at 120° angles, to those two as shown below:, Note that in staggered conformation angle, between bonds 1-A and 2 – A, C, B, is 180°,1 – B and 2 – B is 180°and A, 2, 1 – C and 2 –C is 180°,, 1, i.e. 1-A is parallel to 2 –A ,, A, B, C, 1 – B is parallel to 2 – B and, 1 - C is parallel to 2 – C., Note that in eclipsed conformation angle between, 1 –A and 2 –A is 0°,, A, 1 –B and 2 – B is 0° and, A, 2, 1 – C and 2 – C is 0°,, 1, i.e. 1 – A is parallel to 2 – A,, C, B, 1 – B is parallel to 2 – B and, C, B, 1 – C is parallel to 2 –C., , Dr. R.V.PATIL (HOD), , Mr.R.B. Aochar, , Conformation: A conformation with a 60° dihedral, (torsional) angle is known as staggered, conformation. The angle between the atoms, attached to the front and the rear carbon atoms is, called torsional angle. Eclipse Conformation: A, conformation with 0° torsional angel is known as, eclipsed conformation., H, , H, H, , H, , H, , H, , H, , H, H, H, , H H, , Esclipsed, , Staggered, , OPTICAL ISOMERRISM, , Plane polarized light: Plane polarized light has, vibrations only in one plane. It is obtained by, passing ordinary light through a Nicol prism. It is, made up of calcite or Iceland spar-a crystalline, form of CaCo3., , Optical activity: Compounds which can rotate, the plane of polarized light are called optical active, and the property of a substance to rotate the plane, of polarized light is called as optical activity., , Angle of rotation(α) : It is the angle through, 4. Newman Projection:, Similar to sawhorse, Newman projection represents, the spatial arrangement of all the atoms or groups, on two adjacent carbon atoms. Here the molecule is, viewed along the axis of a carbon- carbon bond.The, carbon atoms toward the front (i.e front carbon) is, represented by a dot and the carbon atoms toward, the rear (i.e, rear carbon) by a circle. The atoms and, groups on the carbon atom are shown as being, bonded to the dot or circle. For example Staggered, , which the plane of polarized light gets rotate when, passes through the solution of an optically active, substance. It depends upon, i)nature of the optically active substance, ii) Concentration of the solution in g /cm3, iii) length of the solution through which light passes, iv) nature of the solvent, v) wavelength of the monochromatic light used, vi) temperature of the sample, The instrument used for measuring the optical, rotation is called a Polarimeter and the light source, is yellow light from a sodium lamp (the sodium Dline with a wavelength of 5893Å) is often used., , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 3
Page 4 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , Dr. R.V.PATIL (HOD), , H3C, , produced when the plane polarized light passes, through one decimeter (1 dm = 10cm) of a solution, of an optically active compound having a unit, concentration at a given wavelength of light (λ) and, at a given temperature (t). it is given by the relation,, Observed angle of, rotation α, Specific rotation, [α] =, ---------------------------------------Length in dm × conc. In g /cm3 solution, l × c, , Chiral objects/molecules. A molecules (or an, object) is said to be chiral or dissymmetric if it is, superimposable on its mirror image and the, property of non-superimposability is called, chirality. Such symmetry or an alternating axis of, symmetry. For example, alphabets (R,S,F,P),, human hand, Shoe or glove,, butanol etc are all chiral object/molecules. Because, do not contain any element of symmetry and hence, mirror image are non-superimposable on their, respective object/molecules., , Achiral objects/molecules. A molecules (or, an object) is said to be achiral if its is, superimposable on its mirror image. such, molecules/object possess some elements of, symmetry (centre of symmetry, plane of symmetry, or an alternating axis of symmetry). For example,, alphabets (A, M and O), dichloromethane, mesotartaric acid etc., are achiral objects/molecules., Because contain a plane of symmetry and hence, their mirror images are superimposable on their, respective object/molecules., , C, , *, , carbon atom which is attached to four different, atoms or group is called a chiral or an asymmetric, carbon atom. Such carbon atom is often marked by, an asterisk (*)., For example,, , Br, , COOH, , C, , *, , H3CH2C, , F, , C, , Lactic acid, , Optical, , *, , CH3, , OH, , I, , OH, , bromochloro, fluoroiodomethane, , butan-2-ol, , isomerism., , Stereoisomers which, resemble one another in their chemical properties, and most of their physical properties but differ in, their behavior towards plane polarized light are, called optical isomers and the phenomenon is called, optical isomerism., , Enantiomers. Stereoisomers which are nonsuperimposable mirror image of, Mirror, each other are called, enantiomers and the, COOH, COOH, phenomenon, HO, C, H, H, C, OH, is called enantiomerism., Enantiomers have, CH3, CH3, identical physical, (+) - Lactic acid (-) - Lactic acid, and chemical properties, (except towards chiral reagents). The enantiomer, which rotates the plane of polarized light toward, right is called dextrorotatory, ie., d or (+) isomer, while the other which rotates the plane of polarized, light towards left is called laevorotatory, ie., l or(-)., For example, lactic acid has two optical isomers, which are enantimers of each other., , Diastereomers. Stereoisomers which are not, mirror image of each other are called diastereomers, and the phenomenon is called diaseoisomerism. For, example, 2,3 dichloropentane exist infour, stereisomeric forms, ie., I, II, III, IV., Mirror, , Mirror, , CH3, , CH3, , CH3, , CH3, , Chiral or Asymmetric carbon atom. A, , H, , Cl, , H, , Specific rotation is the angle of rotation, , Mr.R.B. Aochar, , H, , Cl, , Cl, , H, , H, , Cl, , Cl, , H, , Cl, , H, , H, , Cl, , H, , Cl, , Cl, , H, , CH2CH3, , CH2CH3, , CH2CH3, , CH2CH3, , I, , II, , III, , IV, , I and II are non superimposable mirror image of, each other and hence are enantiomers. similarly, III, and IV. Are enantiomers. On the other hand , I and, III, I and IV, II and III and II and IV are not, mirror image of each other and hence are, diastereomers., Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 4
Page 5 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , and (-) enantiomers is called racemic mixture or, modification. It is denoted by the prefix dl or (±), before the anme of the compound. A racemic, mixture is optically inactive due to external, compensation. Whenever an optically active, compound is synthesized in the laboratory from, achiral reagents, the product is always a racemic, mixture. For example, when 2-butanone is reduced, with usual (achiral reagent) such as Ni/H2 or, LiAlH4, an approximately 50:50 mixture of (+) and, (-) Enantiomers will be formed ie., a racemic, mixture is obtained., , C) Alternating axis of symmetry:A molecule possesses an alternating axis of, symmetry if, when rotated through an angle of 360, 0, / n about this axis and then followed by reflection, in a plane perpendicular to the axis, the molecule is, indistinguishable from the original molecule., Alternating axis of symmetry is rare and it can be, present in cyclic as well as open chain compound., , OH, , CH3CCH2C H3, , Ni/H2 or LiAlH4, , butan-2-one, , HOOC, , H3C, , H, , (±) butan-2-ol, , H, , H, , COOH, , Elements of symmetry, ( three type ) :, HOOC, , H, , COOH, , HOOC, , CH3, , H, , Rotated, 900, , H, , H, , CH3, , H, , HOOC, , H, , H3C, , COOH, , H, , H3C, , H, , H, , H, , CH3, , COOH, , R/S Configurational, Nomenclature:, The order of arrangement of four group around a, chiral carbon is called the absolute configuration, around that atom. System which indicates the, absolute configuration was given by three chemists, R.S. cahn, C.K.Ingold and V.Prelog. This system is, known as (R) and (S) system. The letter (R) comes, from the latin restus (means right or clockwise ), while letter (S) comes from the latin sinister, (means left or anticlockwise)., , Plane of, Symmetry, OH, , OH, , COOH, , Rules to determine the R/S configurational :, Rule 1. If all four atoms directly attached to the, chiral carbon are different, priority depends on their, atomic number. The atom having highest atomic, number gets the highest priority. For example,, , B) Center of symmetry:H3C, , H, , Rotated, 900, , A) Plane of symmetry :-, , HOOC, , A center of symmetry, in molecule is said to exist if a, H, H, H, line I drawn from any atom or H, group to this point and then, extended to equal distance COOH, CH3, beyond this point, meets the, identical atom or group. A center of symmetry is, usually present only in a even number ring., , Rotated, 900, , CH3, , H, , Elements of symmetry offer a simple, device to decide weather a molecule is chiral or, achiral,, i.e. , weather it is superimposible on its mirror, image or not. When a molecule has no plane of, symmetry, no centre of symmetry and alternatig, axis of symmetry, it is nonsuperimposibleon its, mirror image and is chiral (optically active)., , The plane which divides, amolecule into two equal halves H, which are related as object, H, and mirror image is known as, plane of symmetry ., For Example:, , H3C, , H, , CH3CHCH2C H3, , COOH, , Mr.R.B. Aochar, , For example , the molecule of trans 2,4-dimethylcyclobutane-trans-1,3-dicarboxylic acid has a, centre of symmetry., , Racemic mixture. An equimolar mixture of (+), , O, , Dr. R.V.PATIL (HOD), , F, I, , C, Br, , H, , 4, Cl, , 1, , C, 2, , Priority order : I >Br > Cl > F, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , 3, , H3C, , C, D, , 4, Br, , 2, , C, , 1, , 3, , Priority order : Br > -CH3 > D > H, , Page 5
Page 6 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , Rule 2. If the two or more of the atoms directly, bonded to the chiral carbon are identical, the atomic, number of the next atoms are used for priority, assignment. For example,The atoms connected, directly to the chiral carbon are Bromine and three, carbons.Bromine(Br) has the highest priority., Connected to the three carbons are 2H and N, 2H, and Cl, 2H and OH. Chlorine has the highest atomic, number amongst C,H and Cl and thus CH2Cl has, second highest priority. Oxygen in CH2OH has, third priority and Nitrogen in CH2NH2 has last, priority., , Dr. R.V.PATIL (HOD), , Mr.R.B. Aochar, , highest priority to the groups of second and third, priority (ie.,1-2-3 with respect to 4 )the, configuration is designated R., vi)If the arrangement of groups is in anticlockwise, direction is designated S., Example :, Q.1, C, , CH, , C2H5, , Priority order :C, , CH3, , > CH=CHCH3 >, , CH, , C 2 H5, , > CH3, , CH=CHCH3, , Priority order : Br > CH2Cl > CH2OH > CH2NH2, C, C 2H 5, , Br, HOH2C, , C, , C, , CH, , First C H, 2 5, Exchange, , CH3, , Second C H, 2 5, Exchange, , CH=CHCH3, , C, , CH, , CH3, , CH3, , CH=CHCH3, , CH2NH2, , CH=CHCH3, , CH, , S configuration, (Anticlockwise), , CH2Cl, , Q.2, , F, , Rule 3. If a double or a triple b ond is linked to, chiral centre the involved atoms are duplicated or, triplicated respectively., 1), , O, , C, , C, O, , N, , C, , CH, , C, , N, , OH, , C, , OH, , O2 N, , C, , CH, , CN, , >, , C, , CH, , NO2, , First O2N, Exchange, , Second, Exchange, , CN, C, , CN, , O, , N, , >, , F, , O, , O, , NO2, , Priority order : F >, , F, , N, , O, , C, CN, , 3), , 2), , C, , O2 N, , F, , CN, , CH, , C, , CH, , R configuration, (Clockwise), , Steps for designating R/S configuration :, , Q.3, H, , i) Determine the Priority order, ii) The molecules is then visualized so that the, group of lowest priority (4) is directed away, from the observer (at the bottom of the plane), is the first exchange., iii) The remaining three groups are in the plane, facing the observer., iv) In the second exchange, priority order in second, position should be at the top of the plane., v) If the eye travels clockwise as we look from the, group of, , H 2C, , HC, , Br, , Priority order : Br > ph >, , 3, , H, H 2C, , HC, , Br, , First, H2C, Exchange, , Second, HC, , Br Exchange, , Q.4, , Br, , H, , ph, , HC, , CH2, , H, , R configuration, (Clockwise), , H3C, , H, , Br, , Priority order : Br >, , COOH, , >, , CH3, , H, , >, , COOH, COOH, , CH3, , CH3, Br, , First, Exchange, , Br, , HOOC, H, , Second, Exchange, , Br, , CH3, H, , S configuration, (Anticlockwise), , 1, , 4, Dept. Of 4Chemistry, GET’s Science, & Arts college Nagaon, Dhule, Clockwise arrangement, of 1,2,3 and 4 = R, , H, , ph, , 2, 3, , >, , ph, , COOH, , 1, , HC, , ph, , H, , 2, , H2 C, , Anticlockwise arrangement, of 1,2,3 and 4 = S, , Page 6
Page 7 :
Chapter No. 1 Stereoisomerism, Q.5, , ( S.Y.BSc.), , Priority order :, , OH, , >, , >, , CH3, , >, , H, , CH3, OH, , OH, , H, CH3, , Second, , First, Exchange, , CH3 Exchange, , OH, , HO, , H, , CH3, , Q.7, OHC, , Br, I, Cl, , Cl, , H, , Cl, , Q.9, , Q.8, OHC, , CH3, , OH, CH2OH, , H, , 1. abC=Cab : In this type, Two different, substituent, , CH2CH2CH3, OH, H, , C2H5, , a, , a, , Q.10, , CH3, , Cl, , C, , C, , C, , C, b, , b, , H, , H3C, , (Trans), , (Cis), , C, , C, b, , a, , CH3, , H, , C, , C, H, , H, , a, , b, , (Trans), , CH3, , 2. abC=Cad : In this type, Three different, substituent, , Isomerism, , :, , Geometrical isomerism, CH3, H3C, also called as, Cis-Trans isomerism arises due, to the difference in the relative, spatial arrangement of atoms, H, H, or group around the double bond., (Cis)-but-2-ene, In other words,, geometrical isomers have the same, structural formula but differ, CH3, H, in the relative spatial arrangement, of atoms or group around the, double bond.The isomer in which, H, the two similar around atoms or H3C, groups lie on the same side, (Trans)-but-2-ene, of the double bond is called, the cis isomers. The isomer in which the two, similar around atoms or groups lie on the opposite, side of the double bond is called the Trans, isomers. For example,, , a H3C, , a, C C, , d, , b, , CH3, , (Cis), , C C, d, , a, , OH, , H3C, , (Trans), , (Cis), , CH3, , H, , C C, OH, , H, , a, , b, , C C, , (Trans), , 3. abC=Cde :In this type, Four different, COOH, , HOOC, , H, , COOH, , H, , HOOC, , H, , H, , (Trans), Fumaric acid, , (Cis), Maleic acid, , substituent but this compounds of this isomers are, designate as E and Z isomers in order to avoid, uncertainty., e, , a, C, b, , Cl, , H3C, , C, , C, d, , Z-(Cis), (Cis)-but-2-ene, , CH3, , H3C, , (Cis), , GEOMETRICAL ISOMERISM, Geometrical, , The necessary and sufficient condition for a, molecules to exhi bit cis- trans isomerism is that, each carbon atom of the double bond must have two, different atoms or group which may be same or, different. Thus , compounds of the type:, , H, , R configuration, (clockwise), , H, , Mr.R.B. Aochar, , Conditions for geometrical isomerism:, , H, , Q.6, , Dr. R.V.PATIL (HOD), , H, , d, , a, , C, , C, OH, , Z-(Cis), , b, , OH, , H3C, , C, , C, e, , E-(Trans), , H, , C, Cl, , E-(Trans), , (Trans)-but-2-ene, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 7
Page 8 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , Dr. R.V.PATIL (HOD), , Mr.R.B. Aochar, , 2. N=N (Azo compounds), in this type Same, atoms or group in same side by Azo, compounds doublebonds shows Syn while, opposite side shows anti., , E and Z Nomenclature :, E - isomer [Entgegen, German : opposite], Z- isomer [Zusamen, German : same ], 1, , 1, , Cl, , H3C, , N, , 1, , OH, , H3C, , OH, , H, , C, , C, , C, , C, , C6H5, N, , N, C6H5, , C6H5, , C6H5, , anti- Azobenzene, , syn- Azobenzene, , 1, , Cl, , H, , E-(Trans), , Z-(Cis), , Here each olefinic carbon has chloro group of, higher priority while other carbon has methyl, group of higher priority are on the same side it is, the Z-configuration and when they are on the, opposite side it is the E configuration., , Geometrical isomerism in, compounds containing C=N, and N=N bonds, (syn and anti ):, , CONFORMATIONAL, ISOMERISM, The isomers which differ in the conformation are, known as conformational isomers. Conformational, isomers are rapidly interconverted at room, temperature, so they cannot be sparated., Conformational isomers from restricted rotation, about a single bond are also called conformers., , Beside alkenes compounds containing C=N, (aldoximes and Ketoximes) and N=N (Azo, compounds ) bonds also shown geometrical, isomerism. However, in these cases, the prefixes, syn- and anti instead of cis- and Tran are more, frequently used. For example,, , Conformational of Ethane, (CH3—CH3) :, , 1. C=N (aldoximes and Ketoximes) ,, In this type H and OH lie on the same side of, double bond, it is called as syn while anti, configuration they are opposite side, , 2, , Lone pair, , OH, , C6H5, C6H5, , C, , (sp2) orbital, , C, , N, , 1, , N, (sp2) orbital, , H, , 1, , 3, , 3, Front carbon, , Rear carbon, 1,2 and 3 are the three, bonds on the rear carbon, , Eclipsed, , Staggered, , anti- Benzaldoxime, , syn- Benzaldoxime, , C6H5, , Three bonds, present on, front carbon, , 2, , Lone pair, , OH, , H, , N, , CH3, , H3C, N, , N, HO, syn phenyl-anti methyl ketoxime, , C6H5, C, , C, HO, , When an ethane molecules rotates about its carboncarbon single bond, two extreme conformations can, result : the staggered conformation and the, eclipsed conformation. There are several ways to, represent on paper the three dimensional, conformations that occurs as a result of rotation, , anti phenyl-syn methyl ketoxime, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 8
Page 9 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , about a single bond. The carbon that is in the front, is represented by the point at which three bonds, intersect and the carbon that is in the back is, represented by a circle. The three lines emanating, from each of the carbons represent the carbon’s, other three bonds., , Dr. R.V.PATIL (HOD), , substituent. The energy in ethane molecules is 2.9, Kcal/mole. The energy between two conformations, is very small. Thus at room temperature these two, conformations are interconvertible and hence, cannot be isolated., , 60 0, , 00, H, H, H, , H, , Conformational of n-Butane :, (CH3-CH2--CH2-CH3) : CH3 4CH3, , H, , 60 0, Rotation upto 360 0, H, , 1, , H, , H, , Eclipsed, , H2C, , 1CH, , Staggered, , Staggered conformations : A conformation with a, 600 dihedral angle is known as staggered, conformation. The most stable conformation, because the carbon-hydrogen bonds are as far away, from each other as possible. In this case, the, dihedral angle 600,1800 and 3000., Eclipsed conformation : A conformation with a 00, dihedral angle is known as eclipsed conformation., The least stable conformation because in no other, conformation are the carbon-hydrogen bonds closer, to one another. In this case, the dihedral angle, 00,1200 , 2400 and 3600., , 3, , CH3, , H, , H, , CH3, , H, , H, , 600, , 2, , H, H, , HH, , H, H, , H, , (I) 00 or 3600, , (II) 600, , Fully eclipsed, , Gauche, , H, CH3, , (III) 1200, Partially eclipsed, , CH3, , CH3, , CH3, , H, H, , H, , H, , H, CH3, , (IV) 1800, Staggered, , Energy level diagram of ethane molecule :, , 600, , 3, , 3, , CH3, , CH3, , 4, , CH2, , 2, , Rotation at 600 dihedral angle :, , H, , H H, , H, , Mr.R.B. Aochar, , 600, , 0, , 60, , H, , H3C, , H, , H, , H3C, H, , HH, , (V) 2400, Partially eclipsed, , H, , (VI) 3000, Gauche, , Potentional energy, , 1. Staggered or Anti-conformation :, , In this conformation, C2-C3 groups attached to, Maximum apart. The two methyl groups are at 1800, Dihedral angle, hence trosional and the steric strain, is minimum. This makes the staggered, conformation the most stable. The potential energy, is zero Kcal/mole., 00, , 600, , 1200, 1800, 2400, Dihedral angle in degrees, , 3000, , 3600, , The extra energy of the eclipsed conformation is, called torsional strain., Torsional strain is the name give to the repulsion, felt by bonding electron of one substituent as they, pass close the bonding electron of another, , 2. Gauche or Skew conformation :, , In this conformation, the dihedral angle is 600, between two methyl groups. There are non-bonded, interaction betweenTwo methyl groups and it, corresponds to equivalent to 0.8 to 0.9 Kcal/mole., The dihedral angle at 600 and 3000 both are, identical energy. The two are mirror images and are, conformational enantimers., , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 9
Page 10 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , Dr. R.V.PATIL (HOD), Mr.R.B. Aochar, Staggered > Gauche > Partially eclipsed > Fully, eclipsed, , 3. Partially eclipsed conformation :, , In this conformation, two methyl groups are, eclipsing with two non-bonded hydrogen atoms., There are two CH3,H and one H,H torsional, interactions.this increases the potential energy of, the partially eclipsed conformation upto, 3.4 to 3.6 Kcal/mole. This conformation is, comparatively less stable., 2 CH3, H eclipsing = 2 × 1.3 = 2.6 Kcal/mole, 1 H, H eclipsing, = 1 × 1.0 = 1.0 Kcal/mole, Total energy, = 3.6 Kcal/mole, The dihedral angle at 1200 and 2400 both are, identical energy., , STEREOCHEMISTRY OF, CYCLOHEXANE, Conformations, , of, , Cyclohexane, , :, , In, principle, cyclohexane can have a large number of, conformations. out of these, four conformations .,, chair form, boat form, twist boat form and half, chair forms are quite distinct., , 4. Fully eclipsed conformation:, , In this conformation, the two methyl groups are in, close to each other at dihedral angle is 00. Thus ,, there are two H, H eclipsing interactions and one, CH3, CH3 interaction. This conformation has the, highest potential energy 4.4 to 6.1 Kcal/mole and it, is the least preferred., 1 CH3, CH3 eclipsing = 1×2.1 - 4.0 = 2.1 to 4.0, 2 H, H eclipsing = 2 × 1.0, = 2.0 Kcal/mole, Total energy, =, 4.1 to 6.1, Kcal/mole., The dihedral angle, identical energy., , at, , 00 and, , 3600, , both are, , Potentional energy, , 4.1 to 6.1, Kcal/mole, , HALF CHAIR, , CHAIR, , BOAT, , TWIST BOAT, , 1. The chair conformation is more stable than the, boat conformation because of the following two, reasons:, In this chair conformation, all the H-atoms on C1C2, C2-C3, C3-C4, C4-C5, C5-C6 and C6-C1 are in, more stable staggered orientations and hence the, force of attraction between these non-bonded Hatoms is the minimum. on the other hand, in boat, conformation, the adjacent hydrogens on C2-C3 and, C5-C6 are in the less stable eclipsed orientation., These eclipsing interactions raise the energy of the, boat form relative to the chair form., H, , H, , H, , 3.6, Kcal/mole, , H, , H, , H, , 5, , 1, , H, H, H, , 2, , H, , H, , 4, H, , H, , 3, , 5, , 1, 6, , 2, , H, , H, , H, , H, , CHAIR, , 00, , 600, , 1200, 1800, 2400, Dihedral angle in degrees, , 3000, , 3600, , The relation between the potential energy and the, dihedral angle is represented as in energy level, diagram. At room temperature, the kinetic energy is, much higher than the staggered. This conformation, cannot be isolated at room temperature. It has been, estimated that at 250C, 71% of butane molecules, are in staggered conformation while 30% molecules, are in skew or gauche conformation., Stability order:, , H, H, , H, , 0, Kcal/mole, , 4, , 3, , H, , 6, , H, , 0.8 to 0.9, Kcal/mole, , H, , H, , 2. The two hydrogen atoms (marked Hf ) called the, flagpole hydrogens on C1 and C4 in the boat, conformation are quite close (1.83Å or 183, pm) as compared to 2.29Å or 229 pm in the, staggered arrangement, As a result , the, electronic clouds of the σ-bonds of these, hydogens repel each other thereby raising the, H, , Hf, , Hf, , H, 1, , 4, H, , H, H 5, , 6H, H, , H, 3, H, , 2, H, , BOAT, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 10
Page 11 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , energy content futher . thus boat form is less stable, the chair form due to i) eclipsing interaction and ii), flagpole-flagpole interactions. Out of these two, repulsive interactions, flagpole-flagpole interactions, are more severe. The energy difference between the, chair and the boat conformation is 29.7KJ mol-1, , 3. The twist boat (skew boat) conformation is, obtained from the boat conformation by moving the, two flagpole hydrogen little away from each other., As a result, in twist boat conformation, flagpoleflagpole interactions are minimum and hence it is, about 6.7 KJ mol-1 more stable than the boat, formation., , Dr. R.V.PATIL (HOD), , Stability order:, chair > twist boat > Boat > half chair, , Factors Affecting Stability of, Conformations :, 1. Non-Bonded interactions :- Every atom, has a definite atomic size or van der Waal’s, radius. When the two non-bonded atoms, approach each other, the force of attraction, slowly increases. But if non-bonded atoms, come closer instead of attraction, repulsive, forces are set due to space requirement. If the, distance the non-bonded atoms or groups is less, than the sum of their Van der Waal’s, strong, repulsion occurs due to steric crowding. Hence, the staggered conformation is the most stable, and preferred as the non-bonded atoms or, groups are maximum apart. Eclipsed, conformation is least preferred due to strong, steric repulsions., , Hf, Hf, , 1, , 4, , 2, , 5, 3, , 6, , TWIST BOAT, , 4. The half chair conformation is however the, most unstable. In fact it is just a transition state, conformation between the chair and the boat, conformations. The energy difference between the, chair and the half-chair conformation being 44 JK, mol-1 ., , 2. Dipole-Dipole interaction :- it is well, known that the force of repulsion is created, between like charges and the force of attraction, is created between opposite charges. The, conformation, in which dipole-dipole repulsions, are minimum and dipole-dipole attractions are, maximum is preferred. Consider conformation, of ethylene dibromide and ethylene chlorhydrin., The preffered conformation of ethylene bromide, is staggered as it involeves less non-bonded, interaction and the least dipole-dipole, b on d, ogen, repulsions, Hydr, , HALF CHAIR, , As discussed above the energy difference between, the chair and the twist boat conformation is just, 23.0 KJ mol-1.while between the chair and the boat, conformation is 29.7KJ mol-1. This, energy, difference is so small that even at room, temperature the collisions of the molecules supply, sufficient energy to overcome this energy barrier., , Br, , H, , Cl, , Cl, H, , H, , Potential energy, , Mr.R.B. Aochar, , H, Br, , H, , H, , H, , H, OH, , H, O, , H, , H, , H, H, , 44 KJ mol-1, , Staggered ethylene, dibromide, , Staggered, (less stable), , Skew, (more stable), , 29.7KJ mol-1, 6.7 KJ mol-1, , CHAIR, , HALF CHAIR, TWIST BOAT, , BOAT, , HALF CHAIR, TWIST BOAT, , CHAIR, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, Fig. Potential energy relationship among conformations of cyclohexane, , Page 11
Page 12 :
Chapter No. 1 Stereoisomerism, , ( S.Y.BSc.), , In case of ethylene chloridrin the skew of gauche, conformation is most preferred as it is stabilished, by H-bonding., , The bonds which are parallel to the three fold axis, of symmetry of the chair are known as axial bonds, and those which extend outward from the ring are, known as equatorial bonds. Each carbon atom of, cyclohexane has one axial bond and equatorial, bond., H, , H, , H, H, , H, Cyclohexane, , H, , H, , H, , H, , H, , H, , H, , H, , H, H, , H, , H, H, , H, , H, H, , 6 Equatorial Bonds, , H, H, H, , Monosubstituted Cyclohexane :, , Axial bonds, , 6, , H, , H, , 3, , H, CH3, , H, , H, , 4, , 2, , 3, 5, , CH3, , H, , 2, , H, , H, , CH3, , 1, , 6, , H, , H, , Equatorial methyl cyclohexane, , In this case of cyclohexane derivatives when one, hydrogen atom is replaced by a larger group or, atom then the two chair forms are obtained in case, of this monosubstituted derivative. The two, isomeric chair form differ in the position of, substituent. In one isomer the substituent is axially, located whereas in the other, the substituent is, equatorially located. The stabilities of both the, forms are different. Let us consider the case of, methyl cyclohexane. This molecules can have two, isomeric chair forms whose stabilities would be, different. The two isomeric chair forms are, i) Axial isomer, ii) Equatorial isomer., , H, , 1, , H, , 4, , CH3, , 5, , H, H, , H, , H, , H, , H, 6 Axial Bonds, , Newman projection, , Axial methyl cyclohexane, , H, , H, , H, , H, , ii)Equatorial isomer :, , H, , H, , H, H, , H, , H, , H, , Axial and Equatorial Bonds, , H, , 2, , H, , H, , Cyclohexane, , 1, , 6, , H, , H, , 3, , H, , 2, , 3, 5, , H, , H, , 4, , 4, , H, , 1, , 6, , H, , H, , C, , 5, , H, H, , CH3, , H, H, , H, H, , H, , H, , H, H, , H, H, H, , H, , H, , H, , methyl group is located at axial position. The, methyl group is so close to the two axial hydrogens, on the same side of the molecule (attached to, carbon-3 and carbon-5) that the van der waals, forces between them are respulsive. This type of, steric strain, because it arises from an interaction, between Axial group on carbon atoms that have 1,3, relation is called as 1,3-diaxial interaction.1,3diaxial methyl hydrogen interaction is about, 0.9Kcal/mole. The strain caused by a 1,3-diaxial, Interaction in methyl cyclohexane is the same as the, Strain caused by the close proximity of the, hydrogen Atom of methyl group in the gauche from, of butane., , H, , H, , Mr.R.B. Aochar, , i)Axial isomer : In the axial conformer, the, , The Axial and Equatorial Bonds in, Cyclohexane : ., , H, , Dr. R.V.PATIL (HOD), , H, H, , Newman projection, , In the equatorial isomer, the methyl group is, placed at equatorial position . in then equatorial, conformer the methyl group extends into space, away from the rest of molecule because of which, its hydrogen atoms are far away. It is from diaxial, interactions because equatorial methyl group in, anti to carbon -3 and carbon-5., Stability :, Equatorial conformer is, more stable than axial, conformer., , Reference Books:, 1) Organic chemistry - Morrison and Boyd, (6thEdition) 2018, 2) Stereochemistry of organic compounds- E L Eliel, 2008, 3) Stereochemistry of organic compounds- P S Kalsi, 200, , Equtorial bonds, , Dept. Of Chemistry, GET’s Science & Arts college Nagaon, Dhule, , Page 12